Label: APREPITANT capsule
APREPITANT kit
-
NDC Code(s):
68462-112-33,
68462-583-11,
68462-583-40,
68462-583-85, view more68462-584-40, 68462-584-58, 68462-584-76, 68462-585-40, 68462-585-76
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use APREPITANT CAPSULES safely and effectively. See full prescribing information for APREPITANT CAPSULES.
APREPITANT capsules, for oral use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Aprepitant is a substance P/neurokinin 1 (NK1) receptor antagonist.
Aprepitant capsules are indicated
- •
- in combination with other antiemetic agents, in patients 12 years of age and older for prevention of:
- •
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin (1.1)
- •
- nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC) (1.1)
- •
- for prevention of postoperative nausea and vomiting (PONV) in adults (1.2)
Limitations of Use: (1.3)
- •
- Aprepitant has not been studied for treatment of established nausea and vomiting.
- •
- Chronic continuous administration of aprepitant is not recommended.
DOSAGE AND ADMINISTRATION
Recommended Dosage for Prevention of Chemotherapy Induced Nausea and Vomiting (CINV) (2.1)
- •
- Aprepitant capsules in adults and pediatric patients 12 years of age and older: is 125 mg on Day 1 and 80 mg on Days 2 and 3.
- •
- Administer aprepitant 1 hour prior to chemotherapy on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer aprepitant in morning.
- •
- See Full Prescribing Information for recommended dosages of concomitant dexamethasone and 5-HT3 antagonist for HEC and MEC.
Recommended Dosage for PONV (2.2)
- •
- Adults: 40 mg aprepitant capsules within 3 hours prior to induction of anesthesia.
Administration (2.4)
- •
- Aprepitant capsules can be administered with or without food.
- •
- Swallow aprepitant capsules whole.
DOSAGE FORMS AND STRENGTHS
Aprepitant Capsules, USP: 40 mg; 80 mg; 125 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- CYP3A4 Interactions: Aprepitant is a substrate, weak-to-moderate inhibitor and inducer of CYP3A4; See Full Prescribing Information for recommendations regarding contraindications, risk of adverse reactions, and dosage adjustments of aprepitant and concomitant drugs. (4, 5.1, 7.1, 7.2)
- •
- Warfarin (a CYP2C9 substrate): Risk of decreased INR of prothrombin time; monitor INR in 2-week period, particularly at 7 to 10 days, following initiation of aprepitant. (5.2, 7.1)
- •
- Hormonal Contraceptives: Efficacy of contraceptives may be reduced during administration of and for 28 days following the last dose of aprepitant. Use effective alternative or back-up methods of contraception. (5.3, 7.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions are (6.1):
Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
- •
- Adults (≥3%): fatigue, diarrhea, asthenia, dyspepsia, abdominal pain, hiccups, white blood cell count decreased, dehydration, and alanine aminotransferase increased.
- •
- Pediatrics (≥3%): neutropenia, headache, diarrhea, decreased appetite, cough, fatigue, hemoglobin decreased, dizziness, and hiccups.
PONV
- •
- Adults (≥3%): constipation and hypotension.
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
1.2 Prevention of Postoperative Nausea and Vomiting (PONV)
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage For Chemotherapy Induced Nausea and Vomiting (CINV)
2.2 Recommended Dosage For Postoperative Nausea and Vomiting (PONV)
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
5.2 Decrease in INR with Concomitant Warfarin
5.3 Risk of Reduced Efficacy of Hormonal Contraceptives
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Aprepitant on the Pharmacokinetics of Other Drugs
7.2 Effect of Other Drugs on the Pharmacokinetics of Aprepitant
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
14.3 Prevention of Nausea and Vomiting Associated with HEC or MEC in Pediatric Patients
14.4 Prevention of PONV in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
Aprepitant capsules, in combination with other antiemetic agents, are indicated in patients 12 years of age and older for the prevention of:
- •
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- •
- nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage For Chemotherapy Induced Nausea and Vomiting (CINV)
Adults and Pediatric Patients 12 Years of Age and Older
The recommended oral dosage of aprepitant capsules, dexamethasone, and a 5-HT3 antagonist in adults and pediatric patients 12 years of age and older who can swallow oral capsules for the prevention of nausea and vomiting associated with administration of HEC or MEC is shown in Table 1 or Table 2, respectively.
Table 1: Recommended Dosing for the Prevention of Nausea and Vomiting Associated with HEC Population
Day 1
Day 2
Day 3
Day 4
Aprepitant capsules*
Adultsand Pediatric Patients 12 Years and Older
125 mg orally
80 mg orally
80 mg orally
none
Dexamethasone
Adults
12 mg orally
8 mg orally
8 mg orally
8 mg orally
Pediatric Patients 12 Years and Older
If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].
5-HT3 antagonist
Adults and Pediatric Patients 12 Years and Older
See selected 5-HT3 antagonist prescribing information for the recommended dosage
none
none
none
* Administer aprepitant capsules 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer aprepitant capsules in the morning.
† Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with aprepitant [see Clinical Pharmacology (12.3)].
Table 2: Recommended Dosing for the Prevention of Nausea and Vomiting Associated with MEC Population
Day 1
Day 2
Day 3
Aprepitant capsules*
Adults and Pediatric Patients 12 Years and Older
125 mg orally
80 mg orally
80 mg orally
Dexamethasone
Adults
12 mg orally
none
none
Pediatric Patients 12 Years and Older
If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].†
5-HT3 antagonist
Adults and Pediatric Patients 12 Years and Older
See the selected 5-HT3 antagonist prescribing information for recommended dosage
none
none
* Administer aprepitant capsules 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer aprepitant capsules in the morning.
† Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with aprepitant [see Clinical Pharmacology (12.3)].
-
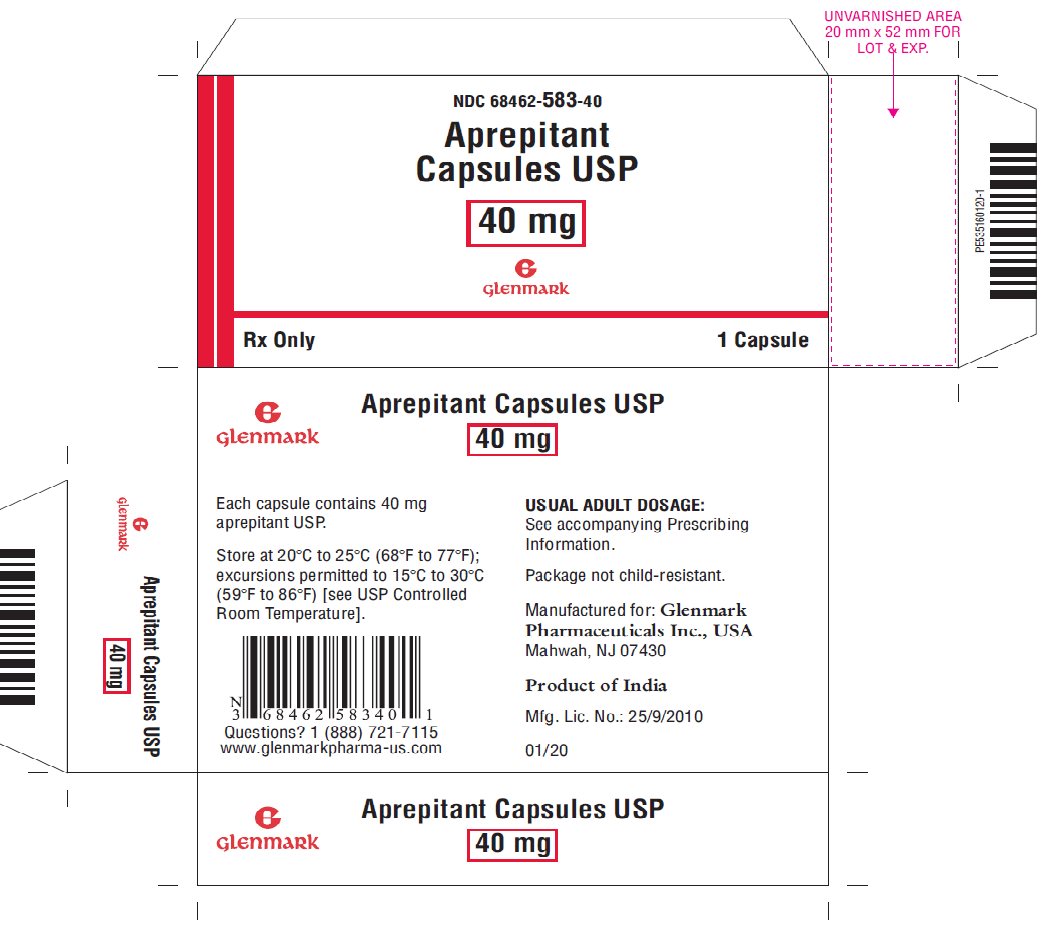
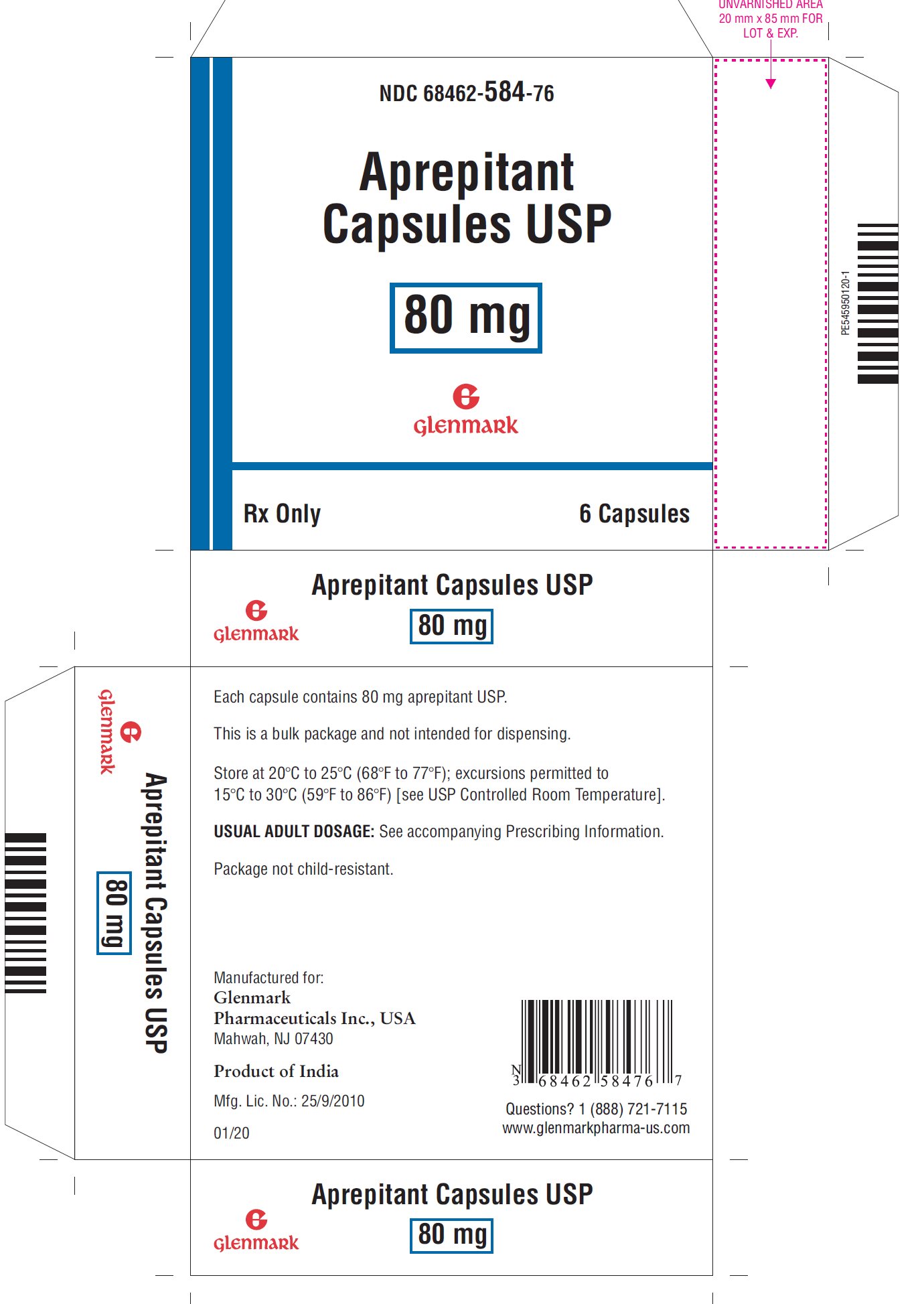
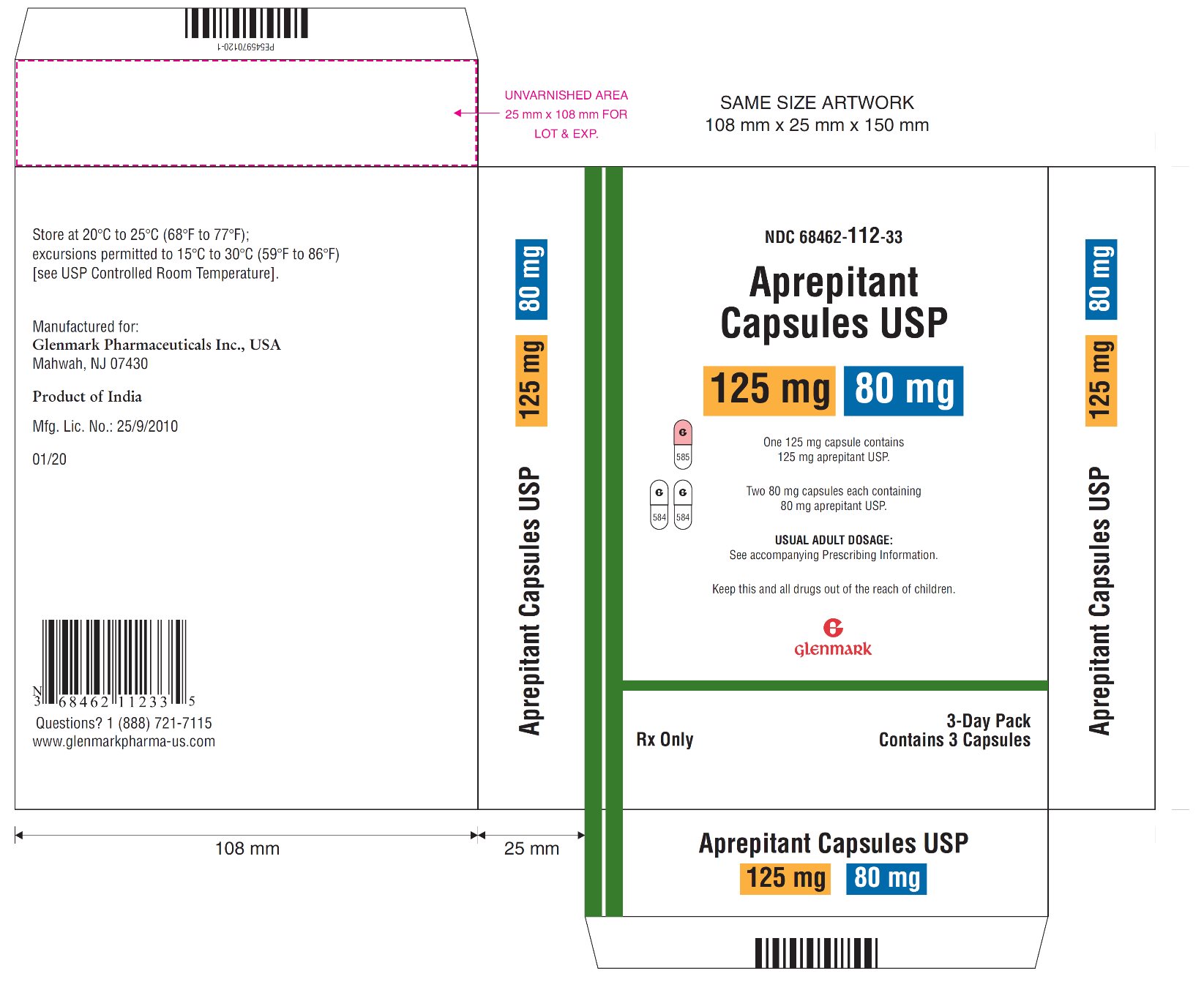
3 DOSAGE FORMS AND STRENGTHS
Aprepitant Capsules, USP:
- •
- 40 mg: hard gelatin capsules with a mustard yellow colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '583' in black ink.
- •
- 80 mg: hard gelatin capsules with a white opaque colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '584' in black ink.
- •
- 125 mg: hard gelatin capsules with a pink opaque colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '585' in black ink.
-
4 CONTRAINDICATIONS
Aprepitant is contraindicated in patients:
- •
- who are hypersensitive to any component of the product. Hypersensitivity reactions including anaphylactic reactions have been reported [see Adverse Reactions (6.2)].
- •
- taking pimozide. Inhibition of CYP3A4 by aprepitant could result in elevated plasma concentrations of this drug which is a CYP3A4 substrate, potentially causing serious or life-threatening reactions, such as QT prolongation, a known adverse reaction of pimozide [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
Aprepitant is a substrate, a weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4.
- •
- Use of aprepitant with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
- •
- Use of pimozide with aprepitant is contraindicated due to the risk of significantly increased plasma concentrations of pimozide, potentially resulting in prolongation of the QT interval, a known adverse reaction of pimozide [see Contraindications (4)].
- •
- Use of aprepitant with strong or moderate CYP3A4 inhibitors (e.g., ketoconazole, diltiazem) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to aprepitant.
- •
- Use of aprepitant with strong CYP3A4 inducers (e.g., rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of aprepitant.
See Table 10 and Table 11 for a listing of potentially significant drug interactions [see Drug Interactions (7.1, 7.2)].
5.2 Decrease in INR with Concomitant Warfarin
Coadministration of aprepitant with warfarin, a CYP2C9 substrate, may result in a clinically significant decrease in International Normalized Ratio (INR) of prothrombin time [see Clinical Pharmacology (12.3)]. Monitor the INR in patients on chronic warfarin therapy in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of aprepitant with each chemotherapy cycle, or following administration of a single 40 mg dose of aprepitant for the prevention of postoperative nausea and vomiting [see Drug Interactions (7.1)].
5.3 Risk of Reduced Efficacy of Hormonal Contraceptives
Upon coadministration with aprepitant, the efficacy of hormonal contraceptives may be reduced during administration of and for 28 days following the last dose of aprepitant [see Clinical Pharmacology (12.3)]. Advise patients to use effective alternative or back-up methods of contraception during treatment with aprepitant and for 1 month following the last dose of aprepitant [see Drug Interactions (7.1), Use in Specific Populations (8.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety of aprepitant was evaluated in approximately 6800 individuals.
Adverse Reactions in Adults in the Prevention of Nausea and Vomiting Associated with HEC and MEC
In 2 active-controlled, double-blind clinical trials in patients receiving highly emetogenic chemotherapy (HEC) (Studies 1 and 2), aprepitant in combination with ondansetron and dexamethasone (aprepitant regimen) was compared to ondansetron and dexamethasone alone (standard therapy) [see Clinical Studies (14.1)].
In 2 active-controlled clinical trials in patients receiving moderately emetogenic chemotherapy (MEC) (Studies 3 and 4), aprepitant in combination with ondansetron and dexamethasone (aprepitant regimen) was compared to ondansetron and dexamethasone alone (standard therapy) [see Clinical Studies (14.2)]. The most common adverse reaction reported in patients who received MEC in pooled Studies 3 and 4 was dyspepsia (6% versus 4%).
Across these 4 studies there were 1412 patients treated with the aprepitant regimen during Cycle 1 of chemotherapy and 1099 of these patients continued into the Multiple-Cycle extension for up to 6 cycles of chemotherapy. The most common adverse reactions reported in patients who received HEC and MEC in pooled Studies 1, 2, 3 and 4 are listed in Table 5.
Table 3: Most Common Adverse Reactions in Patients Receiving HEC and MEC from a Pooled Analysis of HEC and MEC Studies* Aprepitant, ondansetron, and dexamethasone†
(N=1412)Ondansetron and dexamethasone‡
(N=1396)fatigue
13%
12%
diarrhea
9%
8%
asthenia
7%
6%
dyspepsia
7%
5%
abdominal pain
6%
5%
hiccups
5%
3%
white blood cell count decreased
4%
3%
dehydration
3%
2%
alanine aminotransferase increased
3%
2%
*Reported in ≥ 3% of patients treated with the aprepitant regimen and at a greater incidence than standard therapy.
†Aprepitant regimen
‡Standard therapy
In a pooled analysis of the HEC and MEC studies, less common adverse reactions reported in patients treated with the aprepitant regimen are listed in Table 6.
Table 4: Less Common Adverse Reactions in Aprepitant-Treated Patients from a Pooled Analysis of HEC and MEC Studies* Infection and Infestations
oral candidiasis, pharyngitis
Blood and the Lymphatic System Disorders
anemia, febrile neutropenia, neutropenia, thrombocytopenia
Metabolism and Nutrition Disorders
decreased appetite, hypokalemia
Psychiatric Disorders
anxiety
Nervous System Disorders
dizziness, dysgeusia, peripheral neuropathy
Cardiac Disorders
palpitations
Vascular Disorders
flushing, hot flush
Respiratory, Thoracic and Mediastinal Disorders
cough, dyspnea, oropharyngeal pain
Gastrointestinal Disorders
dry mouth, eructation, flatulence, gastritis, gastroesophageal reflux disease, nausea, vomiting
Skin and Subcutaneous Tissue Disorders
alopecia, hyperhidrosis, rash
Musculoskeletal and Connective Tissue Disorders
musculoskeletal pain
General Disorders and Administration Site Condition
edema peripheral, malaise
Investigations
aspartate aminotransferase increased, blood alkaline phosphatase increased, blood sodium decreased, blood urea increased, proteinuria, weight decreased
*Reported in > 0.5% of patients treated with the aprepitant regimen, at a greater incidence than standard therapy and not previously described in Table 5.
In an additional active-controlled clinical study in 1169 patients receiving aprepitant and HEC, the adverse reactions were generally similar to that seen in the other HEC studies with aprepitant.
In another CINV study, Stevens-Johnson syndrome was reported as a serious adverse reaction in a patient receiving the aprepitant regimen with cancer chemotherapy.
Adverse reactions in the Multiple-Cycle extensions of HEC and MEC studies for up to 6 cycles of chemotherapy were generally similar to that observed in Cycle 1.
Adverse Reactions in Pediatric Patients 6 Months to 17 Years of Age in the Prevention of Nausea and Vomiting Associated with HEC or MEC
In a pooled analysis of 2 active-controlled clinical trials in pediatric patients aged 6 months to 17 years who received highly or moderately emetogenic cancer chemotherapy (Study 5 and a safety study, Study 6), aprepitant in combination with ondansetron with or without dexamethasone (aprepitant regimen) was compared to ondansetron with or without dexamethasone (control regimen).
There were 184 patients treated with the aprepitant regimen during Cycle 1 and 215 patients received open-label aprepitant for up to 9 additional cycles of chemotherapy.
In Cycle 1, the most common adverse reactions reported in pediatric patients treated with the aprepitant regimen in pooled Studies 5 and 6 are listed in Table 7.
Table 7: Most Common Adverse Reactions in Aprepitant -Treated Pediatric Patients in HEC and MEC Pooled Studies 5 and 6*
Aprepitant and ondansetron†
(N=184)
Ondansetron‡
(N=168)
neutropenia
13%
11%
headache
9%
5%
diarrhea
6%
5%
decreased appetite
5%
4%
cough
5%
3%
fatigue
5%
2%
hemoglobin decreased
5%
4%
dizziness
5%
1%
hiccups
4%
1%
*Reported in ≥3% of patients treated with the aprepitant regimen and at a greater incidence than control regimen.
† Aprepitant regimen
‡Control regimen
Forty-nine patients were treated with ifosfamide chemotherapy in each arm. Two of the patients treated with ifosfamide in the aprepitant arm developed behavioral changes (agitation = 1; abnormal behavior = 1), whereas no patient treated with ifosfamide in the control arm developed behavioral changes. Aprepitant has the potential for increasing ifosfamide-mediated neurotoxicity through induction of CYP3A4 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Adverse Reactions in Adult Patients in the Prevention of PONV
In 2 active-controlled, double-blind clinical studies in patients receiving general anesthesia (Studies 7 and 8), 40 mg oral aprepitant was compared to 4 mg intravenous ondansetron [see Clinical Studies (14.4)].
There were 564 patients treated with aprepitant and 538 patients treated with ondansetron.
The most common adverse reactions reported in patients treated with aprepitant for PONV in pooled Studies 7 and 8 are listed in Table 8.
Table 5: Most Common Adverse Reactions in Aprepitant-Treated Patients in a Pooled Analysis of PONV Studies* Aprepitant 40 mg
(N = 564)Ondansetron
(N = 538)constipation
9%
8%
hypotension
6%
5%
*Reported in ≥ 3% of patients treated with the aprepitant 40 mg and at a greater incidence than ondansetron.
In a pooled analysis of PONV studies, less common adverse reactions reported in patients treated with aprepitant are listed in Table 9.
Table 6: Less Common Adverse Reactions in Aprepitant-Treated Patients in a Pooled Analysis of PONV Studies* Infections and Infestations
postoperative infection
Metabolism and Nutrition Disorders
hypokalemia, hypovolemia
Nervous System Disorders
dizziness, hypoesthesia, syncope
Cardiac Disorders
bradycardia
Vascular Disorders
hematoma
Respiratory, Thoracic and Mediastinal Disorders
dyspnea, hypoxia, respiratory depression
Gastrointestinal Disorders
abdominal pain, dry mouth, dyspepsia
Skin and Subcutaneous Tissue Disorders
urticaria
General Disorders and Administration Site Conditions
hypothermia
Investigations
blood albumin decreased, bilirubin increased, blood glucose increased, blood potassium decreased
Injury, Poisoning and Procedural Complications
operative hemorrhage, wound dehiscence
*Reported in > 0.5% of patients treated with aprepitant and at a greater incidence than ondansetron
In addition, two serious adverse reactions were reported in PONV clinical studies in patients taking a higher than recommended dose of aprepitant: one case of constipation, and one case of sub-ileus.
Other Studies
Angioedema and urticaria were reported as serious adverse reactions in a patient receiving aprepitant in a non-CINV/non-PONV study (aprepitant is only approved in the CINV and PONV populations).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of aprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Immune system disorders: hypersensitivity reactions including anaphylactic reactions [see Contraindications (4)].
Nervous system disorders: ifosfamide-induced neurotoxicity reported after aprepitant and ifosfamide coadministration.
-
7 DRUG INTERACTIONS
7.1 Effect of Aprepitant on the Pharmacokinetics of Other Drugs
Aprepitant is a substrate, a weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9 [see Clinical Pharmacology (12.3)].
Aprepitant acts as a moderate inhibitor of CYP3A4 when administered as a 3-day regimen (125 mg/80 mg/80 mg) and can increase plasma concentrations of concomitant drugs that are substrates for CYP3A4. Aprepitant acts as a weak inhibitor when administered as a single 40 mg dose and has not been shown to alter the plasma concentrations of concomitant drugs that are primarily metabolized through CYP3A4. Some substrates of CYP3A4 are contraindicated with aprepitant [see Contraindications (4)]. Dosage adjustment of some CYP3A4 and CYP2C9 substrates may be warranted, as shown in Table 10.
Table 7: Effects of Aprepitant on the Pharmacokinetics of Other Drugs CYP3A4 Substrates
Pimozide
Clinical Impact
Increased pimozide exposure.
Intervention
Aprepitant is contraindicated [see Contraindications (4)].
Benzodiazepines
Clinical Impact
Increased exposure to midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)].
Intervention
3-day aprepitant regimen
- •
- Monitor for benzodiazepine-related adverse reactions.
- •
- Depending on the clinical situation (e.g., elderly patients) and degree of monitoring available, reduce the dose of intravenous midazolam
Single 40 mg dose of aprepitant
- •
- No dosage adjustment of the benzodiazepine needed
Dexamethasone
Clinical Impact
Increased dexamethasone exposure [see Clinical Pharmacology (12.3)].
Intervention
3-day aprepitant regimen
- •
- Reduce the dose of oral dexamethasone by approximately 50% [see Dosage and Administration (2.1)].
Single 40 mg dose of aprepitant
- •
- No dosage adjustment of oral dexamethasone needed
Methylprednisolone
Clinical Impact
Increased methylprednisolone exposure [see Clinical Pharmacology (12.3)].
Intervention
3-day aprepitant regimen
- •
- Reduce the dose of intravenous methylprednisolone by approximately 25%
- •
- Reduce the dose of oral methylprednisolone by approximately 50%
Single 40 mg dose of aprepitant
- •
- No dosage adjustment of methylprednisolone needed
Chemotherapeutic agents that are metabolized by CYP3A4
Clinical Impact
Increased exposure of the chemotherapeutic agent may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)].
Intervention
Vinblastine, vincristine, or ifosfamide or other chemotherapeutic agents
- •
- Monitor for chemotherapeutic-related adverse reactions.
Etoposide, vinorelbine, paclitaxel, and docetaxel
- •
- No dosage adjustment needed.
Hormonal Contraceptives
Clinical Impact
Decreased hormonal exposure during administration of and for 28 days after administration of the last dose of aprepitant [see Warnings and Precautions (5.3), Use in Specific Populations (8.3), Clinical Pharmacology (12.3)].
Intervention
Effective alternative or back-up methods of contraception (such as condoms and spermicides) should be used during treatment with aprepitant and for 1 month following the last dose of aprepitant.
Examples
birth control pills, skin patches, implants, and certain IUDs
CYP2C9 Substrates
Warfarin
Clinical Impact
Decreased warfarin exposure and decreased prothrombin time (INR) [see Warnings
Intervention
In patients on chronic warfarin therapy, monitor the prothrombin time (INR) in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day aprepitant regimen with each chemotherapy cycle, or following administration of a single 40 mg dose of aprepitant.
Other
5-HT3 Antagonists
Clinical Impact
No change in the exposure of the 5-HT3 antagonist [see Clinical Pharmacology (12.3)].
Intervention
No dosage adjustment needed
Examples
ondansetron, granisetron, dolasetron
7.2 Effect of Other Drugs on the Pharmacokinetics of Aprepitant
Aprepitant is a CYP3A4 substrate [see Clinical Pharmacology (12.3)]. Co-administration of aprepitant with drugs that are inhibitors or inducers of CYP3A4 may result in increased or decreased plasma concentrations of aprepitant, respectively, as shown in Table 11.
Table 8: Effects of Other Drugs on Pharmacokinetics of Aprepitant Moderate to Strong CYP3A4 Inhibitors
Clinical Impact
Significantly increased exposure of aprepitant may increase the risk of adverse reactions associated with aprepitant [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
Intervention
Avoid concomitant use of aprepitant
Examples
-
Moderate inhibitor:
diltiazem
Strong inhibitors:
ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavirStrong CYP3A4 Inducers
Clinical Impact
Substantially decreased exposure of aprepitant in patients chronically taking a strong CYP3A4 inducer may decrease the efficacy of aprepitant [see Clinical Pharmacology (12.3)].
Intervention
Avoid concomitant use of aprepitant
Examples
rifampin, carbamazepine, phenytoin
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data on use of aprepitant in pregnant women to inform a drug associated risk. In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits exposed during the period of organogenesis to systemic drug levels (AUC) approximately 1.5 times the adult human exposure at the 125 mg/80 mg/80 mg aprepitant regimen [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In embryofetal development studies in rats and rabbits, aprepitant was administered during the period of organogenesis at oral doses up to 1000 mg/kg twice daily in rats and up to the maximum tolerated dose of 25 mg/kg/day in rabbits. No embryofetal lethality or malformations were observed at any dose level in either species. The exposures (AUC) in pregnant rats at 1000 mg/kg twice daily and in pregnant rabbits at 125 mg/kg/day were approximately 1.5 times the adult exposure at the 125 mg/80 mg/80 mg aprepitant regimen. Aprepitant crosses the placenta in rats and rabbits.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted to assess the presence of aprepitant in human milk, the effects on the breastfed infant, or the effects on milk production. Aprepitant is present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for aprepitant and any potential adverse effects on the breastfed infant from aprepitant or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Upon administration of aprepitant, the efficacy of hormonal contraceptives may be reduced. Advise females of reproductive potential using hormonal contraceptives to use an effective alternative or back-up non-hormonal contraceptive (such as condoms and spermicides) during treatment with aprepitant and for 1 month following the last dose [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
8.4 Pediatric Use
Prevention of Nausea and Vomiting Associated with HEC or MEC
The safety and effectiveness of aprepitant capsules in pediatric patients 12 years of age and older for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of HEC, including high-dose cisplatin, and MEC. Use of aprepitant in these age groups is supported by evidence from 95 pediatric patients in a randomized, double-blind, active comparator controlled clinical study (n = 95 patients aged 12 through 17 years). Aprepitant was studied in combination with ondansetron with or without dexamethasone (at the discretion of the physician) [see Clinical Studies (14.3)]. Adverse reactions were similar to those reported in adult patients [see Adverse Reactions (6.1)].
The safety and effectiveness of aprepitant for the prevention of nausea and vomiting associated with HEC or MEC have not been established in patients less than 6 months.
Prevention of Postoperative Nausea and Vomiting (PONV)
The safety and effectiveness of aprepitant have not been established for the prevention of postoperative nausea and vomiting in pediatric patients.
Juvenile Animal Study
A study was conducted in young rats to evaluate the effects of aprepitant on growth and on neurobehavioral and sexual development. Rats were treated at oral doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended pediatric human dose and exposure in female rats equivalent to the pediatric human exposure) from the early postnatal period (Postnatal Day 10) through Postnatal Day 58. Slight changes in the onset of sexual maturation were observed in female and male rats; however, there were no effects on mating, fertility, embryonic-fetal survival, or histomorphology of the reproductive organs. There were no effects in neurobehavioral tests of sensory function, motor function, and learning and memory.
8.5 Geriatric Use
Of the 544 adult cancer patients treated with aprepitant in CINV clinical studies, 31% were aged 65 and over, while 5% were aged 75 and over. Of the 1120 adult cancer patients treated with aprepitant in PONV clinical studies, 7% were aged 65 and over, while 2% were aged 75 and over. Other reported clinical experience with aprepitant has not identified differences in responses between elderly and younger patients. In general, use caution when dosing elderly patients as they have a greater frequency of decreased hepatic, renal or cardiac function and concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
The pharmacokinetics of aprepitant in patients with severe renal impairment and those with end stage renal disease (ESRD) requiring hemodialysis were similar to those of healthy subjects with normal renal function. No dosage adjustment is necessary for patients with any degree of renal impairment or for patients with ESRD undergoing hemodialysis.
8.7 Patients with Hepatic Impairment
The pharmacokinetics of aprepitant in patients with mild and moderate hepatic impairment were similar to those of healthy subjects with normal hepatic function. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9). Therefore, additional monitoring for adverse reactions in these patients may be warranted when aprepitant is administered [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage.
Drowsiness and headache were reported in one patient who ingested 1440 mg of aprepitant (approximately 11 times the maximum recommended single dose).
In the event of overdose, aprepitant should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of aprepitant, drug-induced emesis may not be effective in cases of aprepitant overdosage.
Aprepitant is not removed by hemodialysis.
-
11 DESCRIPTION
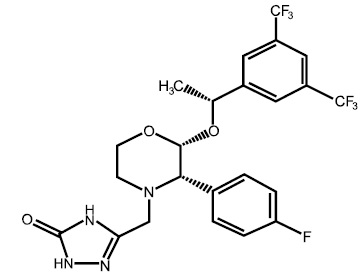
Aprepitant Capsules USP contain the active ingredient aprepitant, USP. Aprepitant, USP is a substance P/neurokinin 1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one.
Its empirical formula is C23H21F7N4O3, and its structural formula is:
Aprepitant, USP is a white to off-white powder, with a molecular weight of 534.43 g/mol. It is soluble in methanol and in acetone, sparingly soluble in ethanol, and practically insoluble in water.
Each capsule for oral administration contains either 40 mg, 80 mg, or 125 mg of aprepitant, USP and the following inactive ingredients: colloidal silicon dioxide, hydroxyethyl cellulose, microcrystalline cellulose, mannitol, poloxamer, povidone, sodium stearyl fumarate, vitamin E polyethylene glycol succinate, and purified water. The capsule shell excipients are gelatin, sodium lauryl sulphate and titanium dioxide. The 40-mg capsule shell also contains iron oxide yellow, and the 125 mg capsule also contains FD&C Red #3. Non-volatile solvents in the imprinting ink are shellac, iron oxide black and potassium hydroxide.
USP dissolution test pending.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting (PONV).
Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies show that aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
12.2 Pharmacodynamics
NK1 Receptor Occupancy
In two single-blind, multiple-dose, randomized, and placebo-controlled studies, healthy young men received oral aprepitant doses of 10 mg (N=2), 30 mg (N=3), 100 mg (N=3) or 300 mg (N=5) once daily (0.08, 0.24, 0.8, and 2.4 times the maximum recommended single dose, respectively) for 14 days with 2 or 3 subjects on placebo. Both plasma aprepitant concentration and NK1 receptor occupancy in the corpus striatum by positron emission tomography were evaluated, at predose and 24 hours after the last dose. At aprepitant plasma concentrations of approximately 10 ng/mL and 100 ng/mL, the NK1 receptor occupancies were approximately 50% and 90%, respectively. The oral aprepitant regimen produced mean trough plasma aprepitant concentrations greater than 500 ng/mL in adults, which would be expected to, based on the fitted curve with the Hill equation, result in greater than 95% brain NK1 receptor occupancy. However, the receptor occupancy has not been determined. In addition, the relationship between NK1 receptor occupancy and the clinical efficacy of aprepitant has not been established.
Cardiac Electrophysiology
In a randomized, double-blind, positive-controlled, thorough QTc study, a single 200-mg dose of fosaprepitant had no effect on the QTc interval. Maximum aprepitant concentrations after a single 200-mg dose of fosaprepitant were 4- and 9-fold higher than that achieved with oral aprepitant 125 mg and 40 mg, respectively. QT prolongation with the oral aprepitant dosing regimens is not expected.
12.3 Pharmacokinetics
Absorption
Following oral administration of a single 40 mg dose of aprepitant in the fasted state, mean area under the plasma concentration-time curve (AUC0-∞) was 7.8 mcg•hr/mL and mean peak plasma concentration (Cmax) was 0.7 mcg/mL, occurring at approximately 3 hours postdose (Tmax). The absolute bioavailability at the 40 mg dose has not been determined.
Following oral administration of a single 125 mg dose of aprepitant on Day 1 and 80 mg once daily on Days 2 and 3, the AUC0-24hr was approximately 19.6 mcg•hr/mL and 21.2 mcg•hr/mL on Day 1 and Day 3, respectively. The Cmax of 1.6 mcg/mL and 1.4 mcg/mL were reached in approximately 4 hours (Tmax) on Day 1 and Day 3, respectively. At the dose range of 80 to 125 mg, the mean absolute oral bioavailability of aprepitant is approximately 60 to 65%. Oral administration of the capsule with a standard high-fat breakfast had no clinically meaningful effect on the bioavailability of aprepitant.
The pharmacokinetics of aprepitant were non-linear across the clinical dose range. In healthy young adults, the increase in AUC0-∞ was 26% greater than dose proportional between 80 mg and 125 mg single doses administered in the fed state.
Distribution
Aprepitant is greater than 95% bound to plasma proteins. The mean apparent volume of distribution at steady state (Vdss) was approximately 70 L in humans.
Aprepitant crosses the blood brain barrier in humans [see Clinical Pharmacology (12.1)].
Elimination
Metabolism
Aprepitant undergoes extensive metabolism. In vitro studies using human liver microsomes indicate that aprepitant is metabolized primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19. Metabolism is largely via oxidation at the morpholine ring and its side chains. No metabolism by CYP2D6, CYP2C9, or CYP2E1 was detected. In healthy young adults, aprepitant accounts for approximately 24% of the radioactivity in plasma over 72 hours following a single oral 300 mg dose of [14C]-aprepitant (2.4 times the maximum recommended dose), indicating a substantial presence of metabolites in the plasma. Seven metabolites of aprepitant, which are only weakly active, have been identified in human plasma.
Excretion
Following administration of a single intravenous 100 mg dose of [14C]-aprepitant prodrug to healthy subjects, 57% of the radioactivity was recovered in urine and 45% in feces. A study was not conducted with radiolabeled capsule formulation. The results after oral administration may differ.
Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. The apparent plasma clearance of aprepitant ranged from approximately 62 to 90 mL/min. The apparent terminal half-life ranged from approximately 9 to 13 hours.
Specific Populations
Geriatric Patients
Following oral administration of a single 125 mg dose of aprepitant on Day 1 and 80 mg once daily on Days 2 through 5 (2 additional days of dosing compared to the recommended duration), the AUC0-24hr of aprepitant was 21% higher on Day 1 and 36% higher on Day 5 in elderly (65 years and older) relative to younger adults. The Cmax was 10% higher on Day 1 and 24% higher on Day 5 in elderly relative to younger adults. These differences are not considered clinically meaningful [see Use in Specific Populations (Error! Hyperlink reference not valid.)].
Pediatric Patients
As part of a 3-day regimen, dosing of aprepitant capsules (125 mg/80 mg/80 mg) in 18 pediatric patients (aged 12 through 17 years) achieved a mean AUC0-24hr of 17 mcg•hr/mL on Day 1 with mean peak plasma concentration (Cmax) at 1.3 mcg/mL occurring at approximately 4 hours. The mean concentrations at the end of Day 2 (N=8) and Day 3 (N=16) were both at 0.6 mcg/mL.
A population pharmacokinetic analysis of aprepitant in pediatric patients (aged 6 months through 17 years) suggests that sex and race have no clinically meaningful effect on the pharmacokinetics of aprepitant.
Male and Female Patients
Following oral administration of a single dose of aprepitant ranging from 40 mg to 375 mg (3 times the maximum aprepitant recommended dose), the AUC0-24hr and Cmax are 9% and 17% higher in females as compared with males. The half-life of aprepitant is approximately 25% lower in females as compared with males and Tmax occurs at approximately the same time. These differences are not considered clinically meaningful.
Racial or Ethnic Groups
Following oral administration of a single dose of aprepitant ranging from 40 mg to 375 mg (3 times the maximum aprepitant recommended dose), the AUC0-24hr and Cmax are approximately 27% and 19% higher in Hispanics as compared with Caucasians. The AUC0-24hr and Cmax were 74% and 47% higher in Asians as compared to Caucasians. There was no difference in AUC0-24hr or Cmax between Caucasians and Blacks. These differences are not considered clinically meaningful.
Patients with Renal Impairment
A single 240 mg dose of aprepitant (approximately 1.9 times the maximum aprepitant recommended dose) was administered to patients with severe renal impairment (creatinine clearance less than 30 mL/min/1.73 m2 as measured by 24-hour urinary creatinine clearance) and to patients with end stage renal disease (ESRD) requiring hemodialysis.
In patients with severe renal impairment, the AUC0-∞ of total aprepitant (unbound and protein bound) decreased by 21% and Cmax decreased by 32%, relative to healthy subjects (creatinine clearance greater than 80 mL/min estimated by Cockcroft-Gault method). In patients with ESRD undergoing hemodialysis, the AUC0-∞ of total aprepitant decreased by 42% and Cmax decreased by 32%. Due to modest decreases in protein binding of aprepitant in patients with renal disease, the AUC of pharmacologically active unbound drug was not significantly affected in patients with renal impairment compared with healthy subjects. Hemodialysis conducted 4 or 48 hours after dosing had no significant effect on the pharmacokinetics of aprepitant; less than 0.2% of the dose was recovered in the dialysate [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Following administration of a single 125 mg dose of aprepitant on Day 1 and 80 mg once daily on Days 2 and 3 to patients with mild hepatic impairment (Child-Pugh score 5 to 6), the AUC0-24hr of aprepitant was 11% lower on Day 1 and 36% lower on Day 3, as compared with healthy subjects given the same regimen. In patients with moderate hepatic impairment (Child-Pugh score 7 to 9), the AUC0-24hr of aprepitant was 10% higher on Day 1 and 18% higher on Day 3, as compared with healthy subjects given the same regimen. These differences in AUC0-24hr are not considered clinically meaningful. There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9) [see Use in Specific Populations (8.7)].
Body Mass Index (BMI)
For every 5 kg/m2 increase in BMI, AUC0-24hr and Cmax of aprepitant decrease by 9% and 10%. BMI of subjects in the analysis ranged from 18 kg/m2 to 36 kg/m2. This change is not considered clinically meaningful.
Drug Interactions Studies
Aprepitant is a substrate, a moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9. Aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter.
Effects of Aprepitant on the Pharmacokinetics of Other Drugs
CYP3A4 substrates (i.e., midazolam): Interactions between aprepitant and coadministered midazolam are listed in Table 12 (increase is indicated as "↑", decrease as "↓", no change as "↔").
Table 9: Pharmacokinetic Interaction Data for Aprepitant and Coadministered Midazolam Dosage of Aprepitant Dosage of Midazolam Observed Drug Interactions Aprepitant 125 mg on Day 1 and 80 mg on Days 2 to 5
oral 2 mg single dose on Days 1 and 5
midazolam AUC ↑ 2.3-fold on Day 1 and ↑ 3.3-fold on Day 5 [see Drug Interactions (7.1)]
Aprepitant 125 mg on Day 1 and 80 mg on Days 2 and 3
intravenous 2 mg prior to 3-day regimen of aprepitant and on Days 4, 8 and 15
midazolam AUC ↑ 25% on Day 4, AUC ↓ 19% on Day 8 and AUC ↓ 4% on Day 15
Aprepitant 125 mg
intravenous 2 mg given 1 hour after aprepitant
midazolam AUC ↑ 1.5-fold
Aprepitant 40 mg
oral 2 mg
midazolam AUC ↑ 1.2-fold on Day 1
A difference of less than 2-fold increase of midazolam AUC is not considered clinically important.
Corticosteroids:
Dexamethasone: Aprepitant, when given as a regimen of 125 mg on Day 1 and 80 mg/day on Days 2 through 5, coadministered with 20 mg dexamethasone on Day 1 and 8 mg dexamethasone on Days 2 through 5, increased the AUC of dexamethasone by 2.2-fold on Days 1 and 5 [see Dosage and Administration (2.1)]. A single dose of aprepitant (40 mg) when coadministered with a single dose of dexamethasone 20 mg, increased the AUC of dexamethasone by 1.45-fold, which is not considered clinically significant.
Methylprednisolone: Aprepitant, when given as a regimen of 125 mg on Day 1 and 80 mg/day on Days 2 and 3, coadministered with 125 mg methylprednisolone IV on Day 1 and 40 mg methylprednisolone orally on Days 2 and 3, increased the AUC of methylprednisolone by 1.34-fold on Day 1 and by 2.5-fold on Day 3.
Chemotherapeutic agents:
Docetaxel: In a pharmacokinetic study, aprepitant (125 mg/80 mg/80 mg regimen) did not influence the pharmacokinetics of docetaxel.
Vinorelbine: In a pharmacokinetic study, aprepitant (125 mg/80 mg/80 mg regimen) did not influence the pharmacokinetics of vinorelbine to a clinically significant degree.
CYP2C9 substrates (Warfarin, Tolbutamide):
Warfarin: A single 125 mg dose of aprepitant was administered on Day 1 and 80 mg/day on Days 2 and 3 to healthy subjects who were stabilized on chronic warfarin therapy. Although there was no effect of aprepitant on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with aprepitant [see Drug Interactions (7.1)].
Tolbutamide: Aprepitant, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered prior to the administration of the 3-day regimen of aprepitant and on Days 4, 8, and 15. This effect was not considered clinically important.
Aprepitant, when given as a 40 mg single dose on Day 1, decreased the AUC of tolbutamide by 8% on Day 2, 16% on Day 4, 15% on Day 8, and 10% on Day 15, when a single dose of tolbutamide 500 mg was administered prior to the administration of aprepitant 40 mg and on Days 2, 4, 8, and 15. This effect was not considered significant.
Other Drugs
Oral contraceptives: When aprepitant was administered as a 3-day regimen (125 mg/80 mg/80 mg) with ondansetron and dexamethasone, and coadministered with an oral contraceptive containing ethinyl estradiol and norethindrone, the trough concentrations of both ethinyl estradiol and norethindrone were reduced by as much as 64% for 3 weeks post-treatment.
P-glycoprotein substrates: Aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter, as demonstrated by the lack of interaction of aprepitant with digoxin in a clinical drug interaction study.
5-HT3 antagonists: In clinical drug interaction studies, aprepitant did not have clinically important effects on the pharmacokinetics of ondansetron, granisetron, or hydrodolasetron (the active metabolite of dolasetron).
Effect of Other Drugs on the Pharmacokinetics of Aprepitant
Ketoconazole: When a single 125 mg dose of aprepitant was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold [see Drug Interactions (7.2)].
Rifampin: When a single 375 mg dose of aprepitant (3 times the maximum aprepitant recommended dose) was administered on Day 9 of a 14-day regimen of 600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold [see Drug Interactions (7.2)].
Diltiazem: In patients with mild to moderate hypertension, administration of aprepitant once daily, as a tablet formulation comparable to 230 mg of the capsule formulation (approximately 1.8 times the aprepitant recommended dose), with diltiazem 120 mg 3 times daily for 5 days, resulted in a 2-fold increase of aprepitant AUC and a simultaneous 1.7-fold increase of diltiazem AUC. These pharmacokinetic effects did not result in clinically meaningful changes in ECG, heart rate or blood pressure beyond those changes induced by diltiazem alone [see Drug Interactions (7.2)].
Paroxetine: Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation (approximately 0.7 and 1.4 times the aprepitant maximum recommended dose), with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine. This effect was not considered clinically important.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat carcinogenicity studies, animals were treated with oral doses ranging from 0.05 to 1000 mg/kg twice daily. The highest dose produced a systemic exposure to aprepitant (AUC) of 0.7 to 1.6 times the adult human exposure at the 125 mg/80 mg/80 mg aprepitant regimen. Treatment with aprepitant at doses of 5 to 1000 mg/kg twice daily caused an increase in the incidences of thyroid follicular cell adenomas and carcinomas in male rats. In female rats, it produced hepatocellular adenomas at 5 to 1000 mg/kg twice daily and hepatocellular carcinomas and thyroid follicular cell adenomas at 125 to 1000 mg/kg twice daily. In the mouse carcinogenicity studies, the animals were treated with oral doses ranging from 2.5 to 2000 mg/kg/day. The highest dose produced a systemic exposure of about 2.8 to 3.6 times the adult human exposure at the 125 mg/80 mg/80 mg aprepitant regimen. Treatment with aprepitant produced skin fibrosarcomas at 125 and 500 mg/kg/day doses in male mice.
Mutagenesis
Aprepitant was not genotoxic in the Ames test, the human lymphoblastoid cell (TK6) mutagenesis test, the rat hepatocyte DNA strand break test, the Chinese hamster ovary (CHO) cell chromosome aberration test and the mouse micronucleus test.
Impairment of Fertility
Aprepitant did not affect the fertility or general reproductive performance of male or female rats at doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended adult human dose and exposure in female rats at about 1.6 times the adult human exposure at the 125 mg/80 mg/80 mg aprepitant regimen).
-
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
Oral administration of aprepitant in combination with ondansetron and dexamethasone (aprepitant regimen) has been shown to prevent acute and delayed nausea and vomiting associated with HEC including high-dose cisplatin, and nausea and vomiting associated with MEC.
In Studies 1 and 2, both multicenter, randomized, parallel, double-blind, controlled clinical studies in adults, aprepitant in combination with ondansetron and dexamethasone was compared with standard therapy (ondansetron and dexamethasone alone) in patients receiving a chemotherapy regimen that included cisplatin greater than 50 mg/m2 (mean cisplatin dose = 80.2 mg/m2). See Table 13.
In these studies, 95% of the patients in the aprepitant group received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common chemotherapeutic agents and the number of aprepitant patients exposed follows: etoposide (106), fluorouracil (100), gemcitabine (89), vinorelbine (82), paclitaxel (52), cyclophosphamide (50), doxorubicin (38), docetaxel (11).
Of the 550 patients who were randomized to receive the aprepitant regimen, 42% were women, 58% men, 59% White, 3% Asian, 5% Black, 12% Hispanic American, and 21% Multi-Racial. The aprepitant-treated patients in these clinical studies ranged from 14 to 84 years of age, with a mean age of 56 years. A total of 170 patients were 65 years or older, with 29 patients being 75 years or older.
Table 10: HEC Treatment Regimens – Studies 1 and 2* Day 1 Day 2 Day 3 Day 4 CINV Aprepitant Regimen
Oral Aprepitant†
125 mg
80 mg
80 mg
none
Oral Dexamethasone‡
12 mg
8 mg
8 mg
8 mg
Ondansetron
5-HT3 antagonist§
none
none
none
CINV Standard Therapy
Oral Dexamethasone
20 mg
8 mg twice daily
8 mg twice daily
8 mg twice daily
Ondansetron
5-HT3 antagonist§
none
none
none
*Aprepitant placebo and dexamethasone placebo were used to maintain blinding.
†Aprepitant was administered 1 hour prior to chemotherapy treatment on Day 1 and in the morning on Days 2 and 3.
‡Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. The 12 mg dose of dexamethasone on Day 1 reflects a dosage adjustment to account for a drug interaction with the aprepitant regimen [see Clinical Pharmacology (12.3)].
§Ondansetron 32 mg intravenous was used in the clinical trials of aprepitant. Although this dose was used in clinical trials, this is no longer the currently recommended dose. Refer to the ondansetron prescribing information for the current recommended dose.
The antiemetic activity of aprepitant was evaluated during the acute phase (0 to 24 hours post-cisplatin treatment), the delayed phase (25 to 120 hours post-cisplatin treatment) and overall (0 to 120 hours post-cisplatin treatment) in Cycle 1. Efficacy was based on evaluation of the following endpoints in which emetic episodes included vomiting, retching, or dry heaves:
- Primary endpoint:
- •
- complete response (defined as no emetic episodes and no use of rescue therapy as recorded in patient diaries)
- Other prespecified endpoints:
- •
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [VAS] score less than 25 mm on a 0 to 100 mm scale)
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- •
- no nausea (maximum VAS less than 5 mm on a 0 to 100 mm scale)
- •
- no significant nausea (maximum VAS less than 25 mm on a 0 to 100 mm scale)
A summary of the key study results from each individual study analysis is shown in Table 14. In both studies, a statistically significantly higher proportion of patients receiving the aprepitant regimen in Cycle 1 had a complete response in the overall phase (primary endpoint), compared with patients receiving standard therapy. A statistically significant difference in complete response in favor of the aprepitant regimen was also observed when the acute phase and the delayed phase were analyzed separately.
Table 11: Percent of Patients Receiving HEC Responding by Treatment Group and Phase — Cycle 1 Study 1
Study 2
ENDPOINTS
Aprepitant Regimen (N=260)*
%Standard Therapy
(N=261)*
%p-Value
Aprepitant Regimen (N=261)*
%Standard Therapy
(N=263)*
%p-Value
PRIMARY ENDPOINT
Complete Response
Overall†
73
52
<0.001
63
43
<0.001
OTHER PRESPECIFIED ENDPOINTS
Complete Response
Acute phase‡
Delayed phase§89
7578
56<0.001
<0.00183
6868
47<0.001
<0.001Complete Protection
Overall
Acute phase
Delayed phase63
85
6649
75
520.001
NS¶
<0.00156
80
6141
65
44<0.001
<0.001
<0.001No Emesis
Overall
Acute phase
Delayed phase78
90
8155
79
59<0.001
0.001
<0.00166
84
7244
69
48<0.001
<0.001
<0.001No Nausea
Overall
Delayed phase48
5144
48NS#
NS#49
5339
40NS¶
NS¶No Significant Nausea
Overall
Delayed phase73
7566
69NS#
NS#71
7364
65NS#
NS#Visual analogue scale (VAS) score range: 0 mm=no nausea; 100 mm=nausea as bad as it could be.
*N: Number of patients (older than 18 years of age) who received cisplatin, study drug, and had at least one post-treatment efficacy evaluation.
†Overall: 0 to 120 hours post-cisplatin treatment.
‡Acute phase: 0 to 24 hours post-cisplatin treatment.
§Delayed phase: 25 to 120 hours post-cisplatin treatment.
¶Not statistically significant when adjusted for multiple comparisons.
#Not statistically significant.
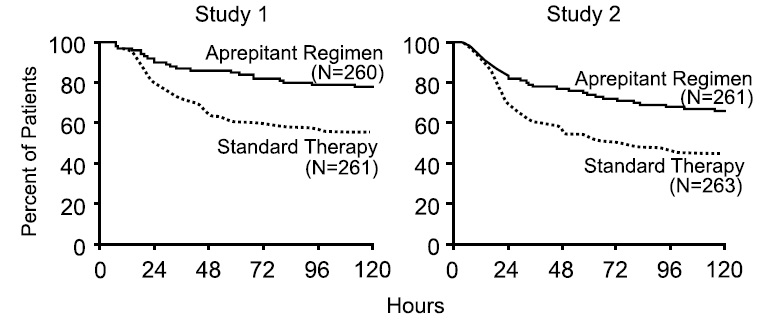
In both studies, the estimated time to first emesis after initiation of cisplatin treatment was longer with the aprepitant regimen, and the incidence of first emesis was reduced in the aprepitant regimen group compared with standard therapy group as depicted in the Kaplan-Meier curves in Figure 1.
Figure 1: Percent of Patients Receiving HEC Who Remain Emesis Free Over Time — Cycle 1
p-Value <0.001 based on a log rank test for Study 1 and Study 2; nominal p-values not adjusted for multiplicity.
Additional Patient-Reported Outcomes: The impact of nausea and vomiting on patients' daily lives was assessed in Cycle 1 of both studies using the Functional Living Index–Emesis (FLIE), a validated nausea-and vomiting-specific patient-reported outcome measure. Minimal or no impact of nausea and vomiting on patients' daily lives is defined as a FLIE total score greater than 108. In each of the 2 studies, a higher proportion of patients receiving the aprepitant regimen reported minimal or no impact of nausea and vomiting on daily life (Study 1: 74% versus 64%; Study 2: 75% versus 64%).
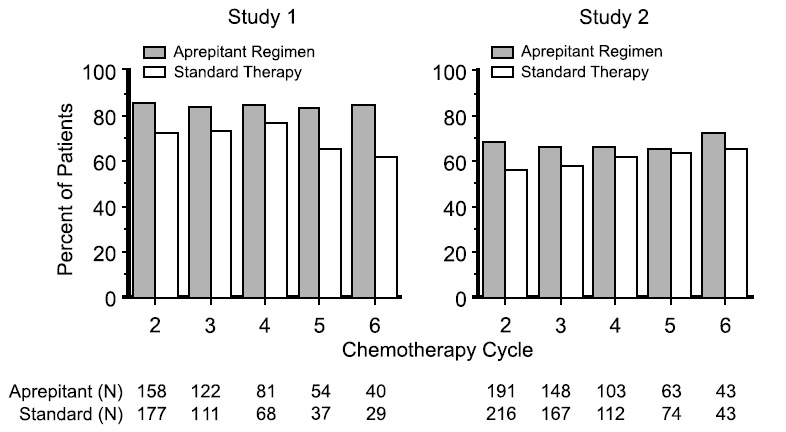
Multiple-Cycle Extension: In the same 2 clinical studies, patients continued into the Multiple-Cycle extension for up to 5 additional cycles of chemotherapy. The proportion of patients with no emesis and no significant nausea by treatment group at each cycle is depicted in Figure 2. Antiemetic effectiveness for the patients receiving the aprepitant regimen was maintained throughout repeat cycles for those patients continuing in each of the multiple cycles.
Figure 2: Proportion of Patients Receiving HEC with No Emesis and No Significant Nausea by Treatment Group and Cycle
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
Aprepitant was studied in two randomized, double-blind, parallel-group studies (Studies 3 and 4) in adult patients receiving MEC.
In Study 3, in breast cancer patients, aprepitant in combination with ondansetron and dexamethasone was compared with standard therapy (ondansetron and dexamethasone) in patients receiving a MEC regimen that included cyclophosphamide 750 to 1500 mg/m2; or cyclophosphamide 500 to 1500 mg/m2 and doxorubicin (less than or equal to 60 mg/m2) or epirubicin (less than or equal to 100 mg/m2). See Table 15.
In this study, the most common combinations were cyclophosphamide + doxorubicin (61%); and cyclophosphamide + epirubicin + fluorouracil (22%).
Of the 438 patients who were randomized to receive the aprepitant regimen, 99.5% were women. Of these, approximately 80% were White, 8% Black, 8% Asian, 4% Hispanic, and less than 1% Other. The aprepitant-treated patients in this clinical study ranged from 25 to 78 years of age, with a mean age of 53 years; 70 patients were 65 years or older, with 12 patients being over 74 years.
Table 12: MEC Treatment Regimens – Studies 3 and 4* Day 1
Day 2
Day 3
CINV Aprepitant Regimen
Oral Aprepitant†
125 mg
80 mg
80 mg
Oral Dexamethasone
12 mg‡
none
none
Oral Ondansetron
8 mg × 2 doses§
none
none
CINV Standard Therapy
Oral Dexamethasone
20 mg‡
none
none
Oral Ondansetron
8 mg × 2 doses§
8 mg twice daily
8 mg twice daily
*Aprepitant placebo and dexamethasone placebo were used to maintain blinding.
†Aprepitant was administered 1 hour prior to chemotherapy treatment on Day 1 and in the mornings on Days 2 and 3.
‡Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1. The 12 mg dose of dexamethasone on Day 1 reflects a dosage adjustment to account for a drug interaction with the aprepitant regimen [see Clinical Pharmacology (12.3)].
§The first ondansetron dose was administered 30 to 60 minutes prior to chemotherapy treatment on Day 1 and the second dose was administered 8 hours after first ondansetron dose.
The antiemetic activity of aprepitant was evaluated based on the following endpoints in which emetic episodes included vomiting, retching, or dry heaves:
- Primary endpoint:
- Other prespecified endpoints:
- •
- complete response (defined as no emetic episodes and no use of rescue therapy as recorded in patient diaries) in the overall phase (0 to 120 hours post-chemotherapy)
- Other prespecified endpoints:
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- •
- no nausea (maximum VAS less than 5 mm on a 0 to 100 mm scale)
- •
- no significant nausea (maximum VAS less than 25 mm on a 0 to 100 mm scale)
- •
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [VAS] score less than 25 mm on a 0 to 100 mm scale)
- •
- complete response during the acute and delayed phases.
A summary of the key results from Study 3 is shown in Table 16. In Study 3, a statistically significantly (p=0.015) higher proportion of patients receiving the aprepitant regimen (51%) in Cycle 1 had a complete response (primary endpoint) during the overall phase compared with patients receiving standard therapy (42%). The difference between treatment groups was primarily driven by the "No Emesis Endpoint", a principal component of this composite primary endpoint. In addition, a higher proportion of patients receiving the aprepitant regimen in Cycle 1 had a complete response during the acute (0 to 24 hours) and delayed (25 to 120 hours) phases compared with patients receiving standard therapy; however, the treatment group differences failed to reach statistical significance, after multiplicity adjustments.
Table 13: Percent of Patients Receiving MEC Responding by Treatment Group and Phase — Cycle 1 of Study 3 ENDPOINTS
Aprepitant
Regimen
(N=433)*
%Standard Therapy
(N=424)*
%
p-Value
PRIMARY ENDPOINT†
Complete Response
51
42
0.015
OTHER PRESPECIFIED ENDPOINTS†
No Emesis
76
59
NS‡
No Nausea
33
33
NS
No Significant Nausea
61
56
NS
No Rescue Therapy
59
56
NS
Complete Protection
43
37
NS
*N: Number of patients included in the primary analysis of complete response.
†Overall: 0 to 120 hours post-chemotherapy treatment.
‡NS when adjusted for prespecified multiple comparisons rule; unadjusted p-value <0.001.
Additional Patient-Reported Outcomes: In Study 3, in patients receiving MEC, the impact of nausea and vomiting on patients' daily lives was assessed in Cycle 1 using the FLIE. A higher proportion of patients receiving the aprepitant regimen reported minimal or no impact on daily life (64% versus 56%). This difference between treatment groups was primarily driven by the "No Vomiting Domain" of this composite endpoint.
Multiple-Cycle Extension: In Study 3, patients receiving MEC were permitted to continue into the Multiple-Cycle extension of the study for up to 3 additional cycles of chemotherapy. The antiemetic effect for patients receiving the aprepitant regimen was maintained during all cycles.
In Study 4, aprepitant in combination with ondansetron and dexamethasone was compared with a standard therapy (ondansetron and dexamethasone alone) in patients receiving a MEC regimen that included any intravenous dose of oxaliplatin, carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, daunorubicin, doxorubicin; cyclophosphamide intravenous (less than 1500 mg/m2); or cytarabine intravenous (greater than 1 g/m2). See Table 15. Patients receiving the aprepitant regimen were receiving chemotherapy for a variety of tumor types including 50% with breast cancer, 21% with gastrointestinal cancers including colorectal cancer, 13% with lung cancer and 6% with gynecological cancers.
Of the 430 patients who were randomized to receive the aprepitant regimen, 76% were women and 24% were men. The distribution by race was 67% White, 6% Black or African American, 11% Asian, and 12% multiracial. Classified by ethnicity, 36% were Hispanic and 64% were non-Hispanic. The aprepitant-treated patients in this clinical study ranged from 22 to 85 years of age, with a mean age of 57 years; approximately 59% of the patients were 55 years or older with 32 patients being over 74 years.
The antiemetic activity of aprepitant was evaluated based on no vomiting (with or without rescue therapy) in the overall period (0 to 120 hours post-chemotherapy) and complete response (defined as no vomiting and no use of rescue therapy) in the overall period.
A summary of the key results from Study 4 is shown in Table 17. In Study 4, a statistically significantly higher proportion of patients receiving the aprepitant regimen (76%) in Cycle 1 had no vomiting during the overall phase compared with patients receiving standard therapy (62%). In addition, a higher proportion of patients receiving the aprepitant regimen (69%) in Cycle 1 had a complete response in the overall phase (0 to 120 hours) compared with patients receiving standard therapy (56%). In the acute phase (0 to 24 hours following initiation of chemotherapy), a higher proportion of patients receiving aprepitant compared to patients receiving standard therapy were observed to have no vomiting (92% and 84%, respectively) and complete response (89% and 80%, respectively). In the delayed phase (25 to 120 hours following initiation of chemotherapy), a higher proportion of patients receiving aprepitant compared to patients receiving standard therapy were observed to have no vomiting (78% and 67%, respectively) and complete response (71% and 61%, respectively).
In a subgroup analysis by tumor type, a numerically higher proportion of patients receiving aprepitant were observed to have no vomiting and complete response compared to patients receiving standard therapy. For sex, the difference in complete response rates between the aprepitant and standard regimen groups was 14% in females (64.5% and 50.3%, respectively) and 4% in males (82.2% and 78.2%, respectively) during the overall phase. A similar difference for sex was observed for the no vomiting endpoint.
Table 14: Percent of Patients Receiving MEC Responding by Treatment Group — Cycle 1 of Study 4 ENDPOINTS
Aprepitant Regimen
(N=430)*
%
Standard Therapy
(N=418)*
%
p-Value
No Vomiting Overall
76
62
<0.0001
Complete Response Overall
69
56
0.0003
*N = Number of patients who received chemotherapy treatment, study drug, and had at least one post-treatment efficacy evaluation.
14.3 Prevention of Nausea and Vomiting Associated with HEC or MEC in Pediatric Patients
In a randomized, double-blind, active comparator-controlled clinical study that included 302 pediatric patients aged 6 months to 17 years receiving HEC or MEC, aprepitant in combination with ondansetron was compared to ondansetron alone (control regimen) for the prevention of CINV (Study 5). Intravenous dexamethasone was permitted as part of the antiemetic regimen in both treatment groups, at the discretion of the physician. A 50% dose reduction of dexamethasone was required for patients in the aprepitant group, reflecting a dosage adjustment to account for a drug interaction [see Clinical Pharmacology (12.3)]. No dexamethasone dose reduction was required for patients who received the control regimen.
Eligible patients had documented malignancy at either an original diagnosis or relapse and were scheduled to receive emetogenic chemotherapy or a chemotherapy regimen not previously tolerated due to vomiting along with ondansetron as part of their antiemetic regimen.
Of the 152 pediatric patients randomized to receive the aprepitant regimen, 55% were male, 45% female, 78% White, 7% Asian, 0% Black, 24% Hispanic, and 13% Multi-Racial. The most common primary malignancies in subjects receiving the aprepitant regimen were osteosarcoma (11%), Ewing’s sarcoma (11%), neuroblastoma (9%) and rhabdomyosarcoma (8%). Other concomitant chemotherapy agents commonly administered and the number of aprepitant patients exposed were: vincristine sulfate (65), etoposide (59), doxorubicin (48), ifosfamide (45), carboplatin (39), and cisplatin (35).
The treatment regimens in Study 5 for pediatric patients are defined in Table 18. Of the pediatric patients, 29% in the aprepitant regimen and 28% in the control regimen used dexamethasone as part of the antiemetic regimen in Cycle 1.
Table 18: HEC and MEC Treatment Regimens* for Pediatric Patients 6 Months to 17 Years of Age— Study 5
Day 1
Day 2
Day 3
CINV aprepitant Regimen
Pediatric Patients 6 Months to less than 12 Years of Age
3 mg/kg body weight oral suspension
2 mg/kg body weight oral suspension
2 mg/kg body weight oral suspension
Pediatric Patients 12 to 17 Years of Age†
125 mg capsule
80 mg capsule
80 mg capsule
Ondansetron
Per standard of care
none
none
CINV Control Regimen
Ondansetron
Per standard of care‡
none
none
- *Intravenous dexamethasone was permitted at the discretion of the physician. A 50% dose reduction of dexamethasone was required for patients in the aprepitant group, reflecting a dosage adjustment to account for a drug interaction [see Clinical Pharmacology (12.3)]. No dexamethasone dose reduction was required for patients in the control regimen.
- †Aprepitant was administered 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy was given on Days 2 and 3, aprepitant was administered in the morning.
‡Ondansetron was administered 30 minutes prior to chemotherapy on Day 1§aprepitant placebo was used to maintain blinding.
The antiemetic activity of aprepitant was evaluated over a 5-day (120 hour) period following the initiation of chemotherapy on Day 1. The primary endpoint in Study 5 was complete response in the delayed phase (25 to 120 hours following chemotherapy) in Cycle 1. Patients had the opportunity to receive open-label aprepitant in subsequent cycles (Optional Cycles 2-6); however, efficacy was not assessed in these optional cycles. Overall efficacy was based on the evaluation of the following endpoints:
Primary endpoint:
- •
- complete response (no vomiting, retching and no use of rescue medication) in the delayed phase (25 to 120 hours following initiation of chemotherapy) Other prespecified endpoints:
- •
- complete response in the acute phase (0 to 24 hours following initiation of chemotherapy)
- •
- complete response in the overall phase (up to 120 hours following initiation of chemotherapy)
- •
- no vomiting (defined as no emesis, retching or dry heaves, regardless of use of rescue medication) in the overall phase
- •
- safety and tolerability
A summary of the key study results are shown in Table 19.
Table 19: Percent of Patients Who Responded to Treatment by Treatment Group and Phase – Cycle 1 of Study 5
Aprepitant
Regimen
n/m (%)
Control
Regimen
n/m (%)
PRIMARY ENDPOINT
Complete Response - Delayed phase
77/152 (50.7)
39/150 (26.0)
OTHER PRESPECIFIED ENDPOINTS
Complete Response* – Acute phase
101/152 (66.4)
78/150 (52.0)
Complete Response* – Overall phase
61/152 (40.1)†
30/150 (20.0)
*Complete Response = No vomiting or retching and no use of rescue medication.
†p<0.01 when compared to Control Regimen
‡ p<0.05 when compared to Control Regimen n/m = Number of patients with desired response/number of patients included in time point. Acute Phase: 0 to 24 hours following initiation of chemotherapy. Delayed Phase: 25 to 120 hours following initiation of chemotherapy. Overall Phase: 0 to 120 hours following initiation of chemotherapy.
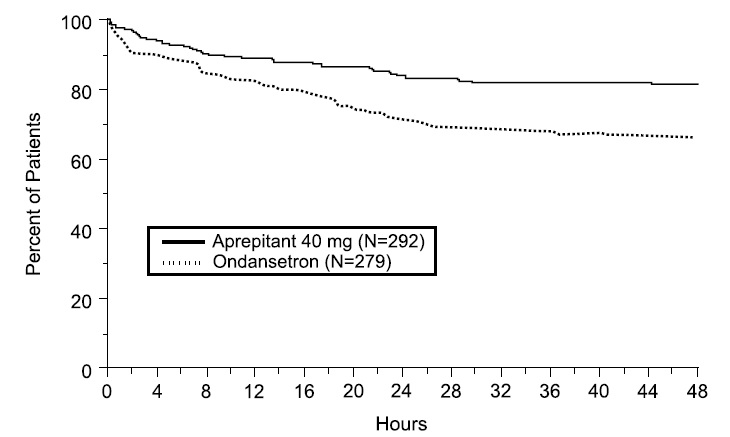
14.4 Prevention of PONV in Adults
In two multicenter, randomized, double-blind, active comparator-controlled, parallel-group clinical studies (Studies 7 and 8), aprepitant was compared with ondansetron for the prevention of postoperative nausea and vomiting in 1658 patients undergoing open abdominal surgery. These two studies were of similar design; however, they differed in terms of study hypothesis, efficacy analyses and geographic location. Study 7 was a multinational study including the U.S., whereas, Study 8 was conducted entirely in the U.S.
In the two studies, patients were randomized to receive 40 mg aprepitant, 125 mg aprepitant, or 4 mg ondansetron as a single dose. Aprepitant was given orally with 50 mL of water 1 to 3 hours before anesthesia. Ondansetron was given intravenously immediately before induction of anesthesia. A comparison between the aprepitant 125 mg dose did not demonstrate any additional clinical benefit over the 40 mg dose and is not a recommended dosage regimen [see Dosage and Administration (2.2)].
Of the 564 patients who received 40 mg aprepitant, 92% were women and 8% were men; of these, 58% were White, 13% Hispanic American, 7% Multi-Racial, 14% Black, 6% Asian, and 2% Other. The age of patients treated with 40 mg aprepitant ranged from 19 to 84 years, with a mean age of 46.1 years. 46 patients were 65 years or older, with 13 patients being 75 years or older.
The antiemetic activity of aprepitant was evaluated during the 0 to 48 hour period following the end of surgery.
- Efficacy measures in Study 7 included:
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- •
- complete response (defined as no emetic episodes and no use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 48 hours following the end of surgery (secondary)
- •
- time to first use of rescue medication in the 0 to 24 hours following the end of surgery (exploratory)
- •
- time to first emesis in the 0 to 48 hours following the end of surgery (exploratory).
A closed testing procedure was applied to control the type I error for the primary endpoints.
The results of the primary and secondary endpoints for 40 mg aprepitant and 4 mg ondansetron are described in Table 20:
Table 20: Response Rates for Select Efficacy Endpoints (Modified-Intention-to-Treat Population) – Study 7
n/m = Number of responders/number of patients in analysis. Δ Difference (%): Aprepitant 40 mg minus Ondansetron. Treatment
n/m (%)
Aprepitant
vs.
Ondansetron
Δ
Odds ratio*
Analysis
PRIMARY ENDPOINTS
No Vomiting 0 to 24 hours (Superiority)
(no emetic episodes)Aprepitant 40 mg
246/293 (84.0)
12.6%
2.1
P<0.001†
Ondansetron
200/280 (71.4)
Complete Response (Non-inferiority: If LB‡ >0.65)
(no emesis and no rescue therapy, 0 to 24 hours)Aprepitant 40 mg
187/293 (63.8)
8.8%
1.4
LB=1.02
Ondansetron
154/280 (55.0)
Complete Response (Superiority: If LB >1.0)
(no emesis and no rescue therapy, 0 to 24 hours)Aprepitant 40 mg
187/293 (63.8)
8.8%
1.4
LB=1.02‡
Ondansetron
154/280 (55.0)
SECONDARY ENDPOINT
No Vomiting 0 to 48 hours (Superiority)
(no emetic episodes)Aprepitant 40 mg
238/292 (81.5)
15.2%
2.3
P<0.001§
Ondansetron
185/279 (66.3)
*Estimated odds ratio for aprepitant versus Ondansetron. A value of >1 favors aprepitant over Ondansetron.
†P-value of two-sided test <0.05.
‡LB = lower bound of 1-sided 97.5% confidence interval for the odds ratio.
§Based on the prespecified fixed sequence multiplicity strategy, aprepitant 40 mg was not superior to Ondansetron.
In Study 7, the use of aprepitant did not affect the time to first use of rescue medication when compared to ondansetron. However, compared to the ondansetron group, use of aprepitant delayed the time to first vomiting, as depicted in Figure 3.
Figure 3: Percent of Patients Who Remain Emesis Free During the 48 Hours Following End of Surgery – Study 7
- Efficacy measures in Study 8 included:
- •
- complete response (defined as no emetic episodes and no use of rescue therapy) in the 0 to 24 hours following the end of surgery (primary)
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 24 hours following the end of surgery (secondary)
- •
- no use of rescue therapy in the 0 to 24 hours following the end of surgery (secondary)
- •
- no emesis (defined as no emetic episodes regardless of use of rescue therapy) in the 0 to 48 hours following the end of surgery (secondary).
Study 8 failed to satisfy its primary hypothesis that aprepitant is superior to ondansetron in the prevention of PONV as measured by the proportion of patients with complete response in the 24 hours following end of surgery.
The study demonstrated that 40 mg aprepitant had a clinically meaningful effect with respect to the secondary endpoint "no vomiting" during the first 24 hours after surgery and was associated with a 16% improvement over ondansetron for the no vomiting endpoint.
Table 21: Response Rates for Select Efficacy Endpoints (Modified-Intention-to-Treat
Population) – Study 8
Treatment
n/m (%)
Aprepitant
vs.
Ondansetron
Δ
Odds ratio*
Analysis
PRIMARY ENDPOINT
Complete Response
(no emesis and no rescue therapy, 0 to 24 hours)Aprepitant 40 mg
111/248 (44.8)
2.5%
1.1
0.61
Ondansetron
104/246 (42.3)
SECONDARY ENDPOINTS
No Vomiting
(no emetic episodes, 0 to 24 hours)Aprepitant 40 mg
223/248 (89.9)
16.3%
3.2
<0.001†
Ondansetron
181/246 (73.6)
No Use of Rescue Medication
(for established emesis or nausea, 0 to 24 hours)Aprepitant 40 mg
112/248 (45.2)
-0.7%
1.0
0.83
Ondansetron
113/246 (45.9)
No Vomiting 0 to 48 hours (Superiority)
(no emetic episodes, 0 to 48 hours)Aprepitant 40 mg
209/247 (84.6)
17.7%
2.7
<0.001*
Ondansetron
164/245 (66.9)
n/m = Number of responders/number of patients in analysis.
Δ Difference (%): Aprepitant 40 mg minus Ondansetron.
*Estimated odds ratio: Aprepitant 40 mg versus Ondansetron.
†Not statistically significant after pre-specified multiplicity adjustment.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Aprepitant Capsules USP, 125 mg: hard gelatin capsules with a pink opaque colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '585' in black ink. They are supplied as follows:
- NDC 68462-585-76 Carton of 6 capsules (containing 1 x 6 unit-dose blisters)
Aprepitant Capsules USP, 80 mg: hard gelatin capsules with a white opaque colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '584' in black ink. They are supplied as follows:
- NDC 68462-584-76 Carton of 6 capsules (containing 1 x 6 unit-dose blisters)
- NDC 68462-584-58 Blister card of 2 capsules (2-day pack containing two 80 mg capsules)
Aprepitant Capsules USP, 3-day pack (125 mg/80 mg/80 mg):
- NDC 68462-112-33 Blister card of 3 capsules (3-day pack containing one 125 mg capsule and two 80 mg capsules)
Aprepitant Capsules USP, 40 mg: hard gelatin capsules with a mustard yellow colored cap imprinted with a Glenmark logo 'G' in black ink and a white opaque colored body imprinted with '583' in black ink. They are supplied as follows:
- NDC 68462-583-11 Carton of 10 capsules (containing 1 x 10 unit-dose blisters)
- NDC 68462-583-85 Carton of 5 capsules (containing 5 x 1 unit-dose blisters)
- NDC 68462-583-40 Carton of 1 capsule (containing 1 unit-dose blisters)
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions, including anaphylaxis, have been reported in patients taking aprepitant. Advise patients to stop taking aprepitant and seek immediate medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as hives, rash and itching, skin peeling or sores, or difficulty in breathing or swallowing.
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription, non-prescription medication or herbal products [see Contraindications (4), Warnings and Precautions (5.1)].
Warfarin: Instruct patients on chronic warfarin therapy to follow instructions from their healthcare provider regarding blood draws to monitor their INR during the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of aprepitant with each chemotherapy cycle, or following administration of a single 40-mg dose of aprepitant for the prevention of postoperative nausea and vomiting [see Warnings and Precautions (5.2)].
Hormonal Contraceptives: Advise patients that administration of aprepitant may reduce the efficacy of hormonal contraceptives. Instruct patients to use effective alternative or back-up methods of contraception (such as condoms and spermicides) during treatment with aprepitant and for 1 month following the last dose of aprepitant [see Warnings and Precautions (5.3), Use in Specific Populations (8.3)].
Manufactured by:
Manufactured by:
Glenmark Pharmaceuticals Limited
Plot No. 2, Phase-2, Pharma Zone SEZ
Pithampur, Dist.-Dhar
Madhya Pradesh 454 775, India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Product of India
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
January 2020
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: January 2020
- Package/Label Display Panel
- Package/Label Display Panel
- Package/Label Display Panel
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
APREPITANT
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68462-583 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APREPITANT (UNII: 1NF15YR6UY) (APREPITANT - UNII:1NF15YR6UY) APREPITANT 40 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color YELLOW (mustard [cap]) , WHITE (opaque [body]) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code G;583 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68462-583-11 1 in 1 CARTON 10/12/2017 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:68462-583-85 5 in 1 CARTON 10/12/2017 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:68462-583-40 1 in 1 CARTON 10/12/2017 3 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 APREPITANT
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68462-584 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APREPITANT (UNII: 1NF15YR6UY) (APREPITANT - UNII:1NF15YR6UY) APREPITANT 80 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (opaque [cap]) , WHITE (opaque [body]) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code G;584 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68462-584-58 2 in 1 DOSE PACK; Type 0: Not a Combination Product 10/12/2017 2 NDC:68462-584-76 1 in 1 CARTON 10/12/2017 2 NDC:68462-584-40 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 APREPITANT
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68462-585 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APREPITANT (UNII: 1NF15YR6UY) (APREPITANT - UNII:1NF15YR6UY) APREPITANT 125 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color PINK (opaque [cap]) , WHITE (opaque [body]) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G;585 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68462-585-76 1 in 1 CARTON 10/12/2017 1 NDC:68462-585-40 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 APREPITANT
aprepitant kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68462-112 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68462-112-33 1 in 1 DOSE PACK; Type 0: Not a Combination Product 10/12/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 2 Part 2 1 BLISTER PACK 1 Part 1 of 2 APREPITANT
aprepitant capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APREPITANT (UNII: 1NF15YR6UY) (APREPITANT - UNII:1NF15YR6UY) APREPITANT 80 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (opaque [cap]) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code G;584 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 Part 2 of 2 APREPITANT
aprepitant capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APREPITANT (UNII: 1NF15YR6UY) (APREPITANT - UNII:1NF15YR6UY) APREPITANT 125 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color PINK (opaque [cap]) , WHITE (opaque [body]) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G;585 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207777 10/12/2017 Labeler - Glenmark Pharmaceuticals Inc., USA (130597813) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals Limited 862603186 MANUFACTURE(68462-583, 68462-584, 68462-585, 68462-112) , ANALYSIS(68462-583, 68462-584, 68462-585, 68462-112)