Label: DOCUSATE SODIUM capsule
- NDC Code(s): 50804-862-25
- Packager: Good Sense (Geiss, Destin & Dunn, Inc.)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
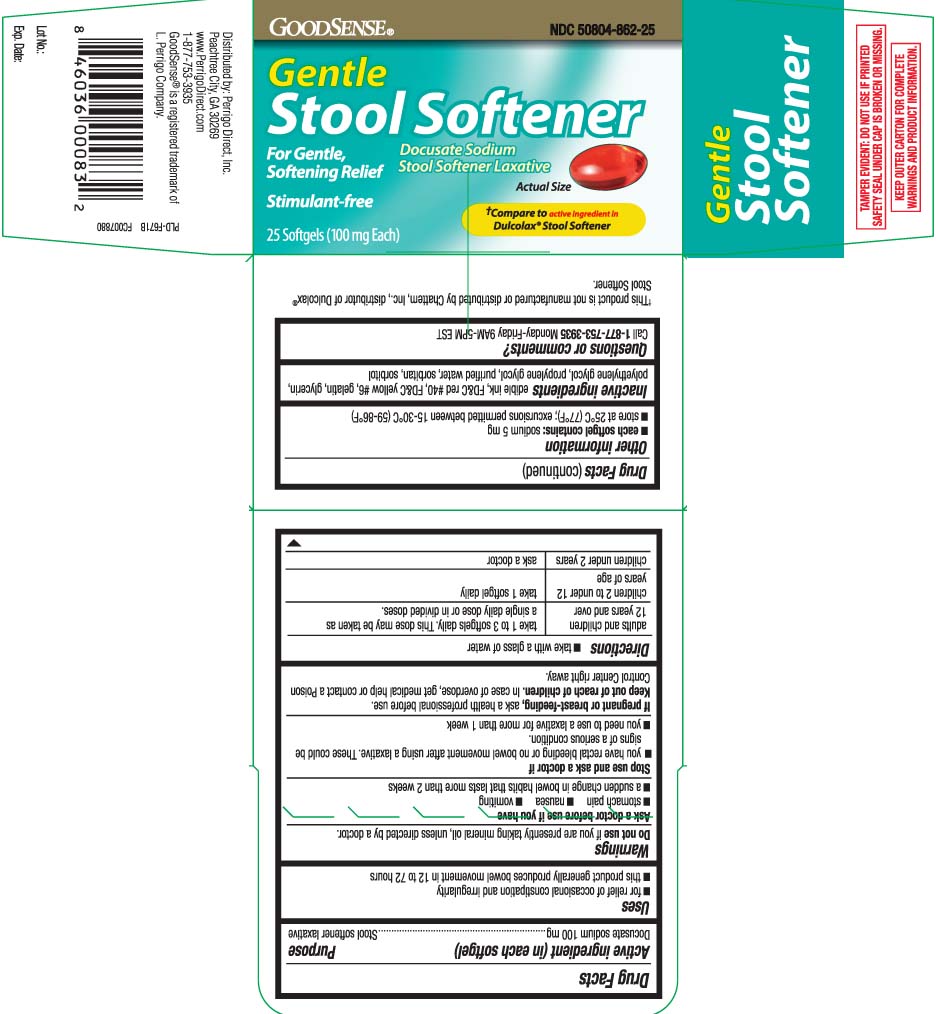
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that last over 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
†Compare to the active ingredient in Dulcolax® Stool Softener
Gentle
Stool Softener
Docusate Sodium
Stool Softener Laxative
For Gentle Softener Relief
Stimulant-free
Softgels
†This product is not manufactured or distributed by Chattem, Inc., distributor of Dulcolax
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: Perrigo Direct, Inc.
Peachtree City, GA 30269
- Product Label
-
INGREDIENTS AND APPEARANCE
DOCUSATE SODIUM
docusate sodium capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50804-862 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) SORBITAN (UNII: 6O92ICV9RU) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code P51 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50804-862-25 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/28/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 05/28/2021 Labeler - Good Sense (Geiss, Destin & Dunn, Inc.) (076059836)