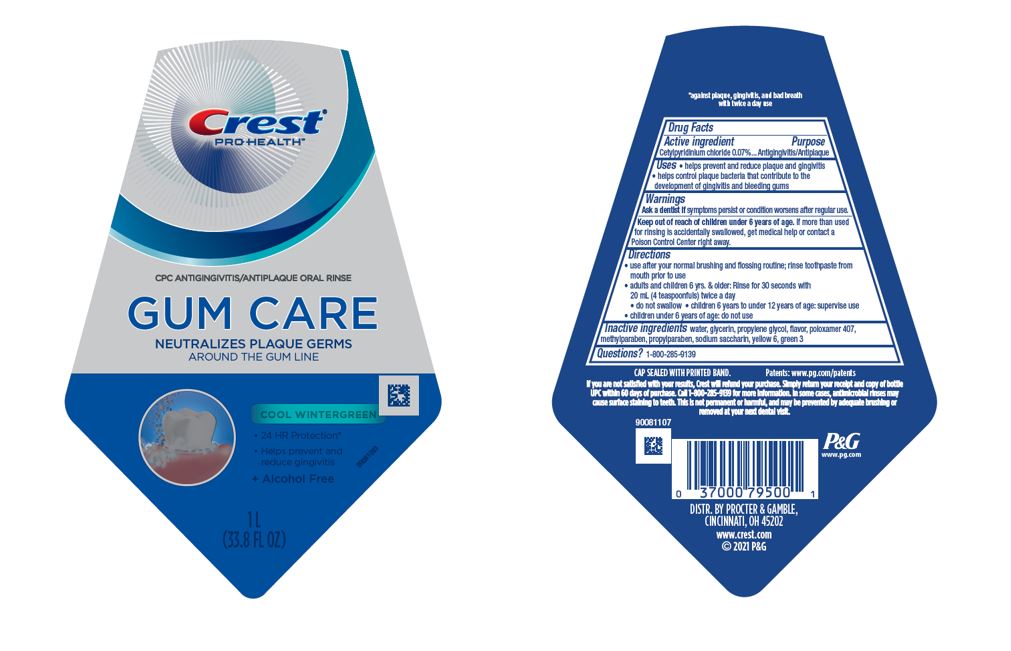
Label: CREST PRO-HEALTH GUM CARE- cetylpyridinium chloride rinse
- NDC Code(s): 69423-864-16, 69423-864-33, 69423-864-67
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1 L Bottle Label
-
INGREDIENTS AND APPEARANCE
CREST PRO-HEALTH GUM CARE
cetylpyridinium chloride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-864 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.07 g in 1 mL Inactive Ingredients Ingredient Name Strength POLOXAMER 407 (UNII: TUF2IVW3M2) WATER (UNII: 059QF0KO0R) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) Product Characteristics Color green Score Shape Size Flavor WINTERGREEN Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-864-33 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/03/2022 2 NDC:69423-864-16 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/03/2022 3 NDC:69423-864-67 2 in 1 CELLO PACK 02/03/2022 3 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 02/03/2022 Labeler - The Procter & Gamble Manufacturing Company (004238200)