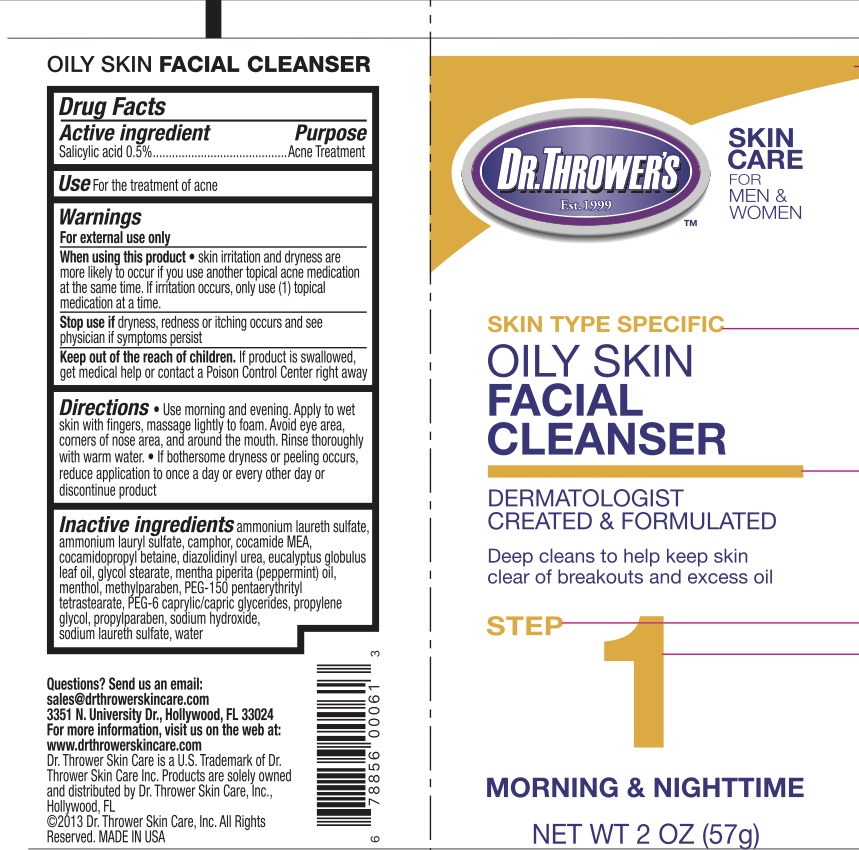
Label: DR THROWERS OILY SKIN FACIAL CLEANSER- salicylic acid 2% liquid
- NDC Code(s): 72839-613-02
- Packager: Derma Care Research Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Stop use if dryness, redness, or itching occurs and see a physician if symptoms persist.
- Keep Out of Reach of Children.
- Directions
-
Inactive Ingredients
Ammonium laureth sulfate, ammonium lauryl sulfate, camphor, cocamide MEA, cocamidopropyl betaine, diazolidinyl urea, eucalyptus globulus leaf oil, glycol stearate, mentha piperita (peppermint) oil, menthol, methylparaben, PEG-150 pentaerythrityl tetrastearate, PEG-6 caprylic/capric glycerides, propylene glycol, propylparaben, sodium hydroxide, sodium laureth sulfate, water.
- Label
-
INGREDIENTS AND APPEARANCE
DR THROWERS OILY SKIN FACIAL CLEANSER
salicylic acid 2% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72839-613 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength PEG-6 CAPRYLIC/CAPRIC GLYCERIDES (UNII: GO50W2HWO8) GLYCOL STEARATE (UNII: 0324G66D0E) PEPPERMINT OIL (UNII: AV092KU4JH) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) COCO MONOETHANOLAMIDE (UNII: C80684146D) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EUCALYPTUS OIL (UNII: 2R04ONI662) PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) MENTHOL (UNII: L7T10EIP3A) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72839-613-02 57 g in 1 TUBE; Type 0: Not a Combination Product 09/28/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 09/28/2021 Labeler - Derma Care Research Labs, LLC (116817470)