Label: SPORIMUNE- cyclosporine capsule
- NDC Code(s): 17033-261-15, 17033-262-15, 17033-263-15
- Packager: Dechra Veterinary Products LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated September 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Caution:
-
Description:
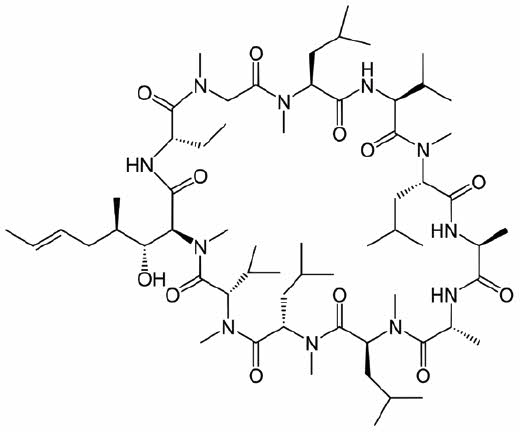
Sporimune (cyclosporine capsules) USP MODIFIED is an oral form of cyclosporine that immediately forms a microemulsion in an aqueous environment. Cyclosporine, the active ingredient in Sporimune is a cyclic polypeptide, immune modulating agent consisting of 11 amino acids. It is produced as a metabolite by the fungal species Beauveria nivea.
Chemically, cyclosporine A is designated Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)- 6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-ananyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl].
The structural formula is:
- Indications:
-
Dosage and Administration:
The initial dose of Sporimune is 5 mg/kg/day (3.3-6.7 mg/kg/day) as a single daily dose for 30 days. Following this initial daily treatment period, the dose of Sporimune may be tapered by decreasing the frequency of dosing to every other day or twice weekly, until a minimum frequency is reached which will maintain the desired therapeutic effect. Sporimune should be given at least one hour before or two hours after a meal. If a dose is missed, the next dose should be administered (without doubling) as soon as possible, but dosing should be no more frequent than once daily.
Dose Administration Dog body weight (lbs) Dog body weight (kg) Dose 5 mg/kg 4 – 6.5 lbs 2 – 2.9 kg 10 mg capsule 6.6 – 9 lbs 3 – 3.9 kg 2 × 10 mg capsules 9.1 – 16 lbs 4 – 7.9 kg 25 mg capsule 16.1 – 33 lbs 8 – 14.9 kg 50 mg capsule 33.1 – 64 lbs 15 – 28.9 kg 100 mg capsule 64.1 – 79 lbs 29 – 35.9 kg 100 mg capsule +50 mg capsule 79.1 – 121 lbs 36 – 55.9 kg 2 × 100 mg capsules - Contraindications:
- Warnings:
-
Human Warnings:
Not for human use. Keep this and all drugs out of reach of children.
For use only in dogs.
Capsules should not be broken or opened. Wear gloves during administration.
Wash hands after administration. In case of accidental ingestion, seek medical advice immediately and provide the package insert or the label to the physician.
-
Precautions:
The safety and effectiveness of Sporimune has not been established in dogs less than 6 months of age or less than 4 lbs body weight. Sporimune is not for use in breeding dogs, pregnant or lactating bitches.
As with any immunomodulation regimen, exacerbation of sub-clinical neoplastic and infectious conditions may occur.
Gastrointestinal problems and gingival hyperplasia may occur at the initial recommended dose (See Animal Safety).
Sporimune may cause elevated levels of serum glucose, and should be used with caution in cases with diabetes mellitus. If signs of diabetes mellitus develop following the use of Sporimune consideration should be given to tapering or discontinuing the dose.
Sporimune should be used with caution with drugs that affect the P-450 enzyme system. Simultaneous administration of Sporimune with drugs that suppress the P-450 enzyme system, such as azoles (e.g. ketoconazole), may lead to increased plasma levels of cyclosporine.
Since the effect of cyclosporine use on dogs with compromised renal function has not been studied, Sporimune should be used with caution in dogs with renal insufficiency.
There have been reports of convulsions in human adult and pediatric patients receiving cyclosporine, particularly in combination with high dose methylprednisolone (See Animal Safety).
Killed vaccines are recommended for dogs receiving Sporimune because the impact of cyclosporine on the immune response to modified live vaccines is unknown (See Animal Safety).
-
Adverse Reactions:
A total of 265 dogs were included in the field study safety analysis. One hundred and eleven (111) dogs were treated with placebo for the first 30 days. For the remainder of the study, all dogs received cyclosporine capsules.
Fourteen dogs withdrew from the study due to adverse reactions. Four dogs withdrew from the study after vomiting. One dog each withdrew from the study after diarrhea; vomiting, diarrhea and pruritus; vomiting, depression and lethargy; lethargy, anorexia and hepatitis; gingival hyperplasia, lethargy, polyuria/polydipsia and soft stool; seizure; sebaceous cyst; pruritus; erythema; or otitis externa.
Vomiting and diarrhea were the most common adverse reactions occurring during the study. In most cases, signs spontaneously resolved with continued dosing. In other cases, temporary dose modifications (brief interruption in dosing, divided dosing, or administration with a small amount of food) were employed to resolve signs.
Persistent otitis externa, urinary tract infections, anorexia, gingival hyperplasia, lymphadenopathy and lethargy were the next most frequent adverse events observed. Gingival hyperplasia regressed with dose tapering. Owners of four dogs reported seizures while dogs were receiving cyclosporine. In one dog, seizures were the result of a brain tumor diagnosed one month into the study. Another dog experienced seizures before and after the study.
Otitis externa, allergic otitis, or pinna erythema, with or without exudates, commonly accompanies atopy. Many dogs entered the study with otitis externa, which did not resolve without otic treatment. New cases of otitis externa, allergic otitis, or pinna erythema developed while dogs were receiving cyclosporine. However, the incidence rate was lower with cyclosporine compared to placebo. A change in the dose frequency was not necessary when new cases occurred.
Number of Dogs Displaying Each Clinical Observation in the Field Study Clinical Sign % out of 265 Vomiting 30.9% Diarrhea 20.0% Persistent Otitis Externa 6.8% Urinary Tract Infection 3.8% Anorexia 3.0% Lethargy 2.3% Gingival Hyperplasia 2.3% Lymphadenopathy 2.3% The following clinical signs were reported in less than 2% of dogs treated with cyclosporine in the field study: constipation, flatulence, Clostridial organisms in the feces, nausea, regurgitation, polyuria/polydipsia, strong urine odor, proteinuria, pruritus, erythema/flushed appearance, pyoderma, sebaceous adenitis, crusty dermatitis, excessive shedding, coarse coat, alopecia, papillomas, histiocytoma, granulomatous mass or lesion, cutaneous cyst, epulis, benign epithelial tumor, multiple hemangioma, raised nodule on pinna, seizure, shaking/trembling, hind limb twitch, panting, depression, irritability, hyperactivity, quieter, increased light sensitivity, reluctance to go outside, weight loss, hepatitis.
The following clinical signs were observed in 1.5-4.5% of dogs while receiving the placebo: vomiting, diarrhea and urinary tract infection. The following clinical signs were observed in less than 1% of dogs receiving the placebo: anorexia, otitis externa, cutaneous cysts, corneal opacity, lymphadenopathy, erythema/flushed appearance.
Clinical Pathology Changes: During the study, some dogs experienced changes in clinical chemistry parameters while receiving cyclosporine, as described in the following table:
Clinical Chemistry % Affected (out of 265) Elevated Creatinine 7.8% Hyperglobulinemia 6.4% Hyperphosphatemia 5.3% Hyperproteinemia 3.4% Hypercholesterolemia 2.6% Hypoalbuminemia 2.3% Hypocalcemia 2.3% Elevated BUN 2.3% In addition, the following changes in clinical chemistry parameters were noted in less than 2% of dogs: hypernatremia; hyperkalemia, elevated ALT, elevated ALP, hypercalcemia and hyperchloremia. These clinical pathology changes were generally not associated with clinical signs.
Post-approval Experience: (Rev 2014)
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are grouped by body system and are presented in decreasing order of reporting frequency.
Gastrointestinal: Emesis, diarrhea, gingival hyperplasia, hemorrhagic diarrhea, abdominal pain, hematemesis, digestive tract hemorrhage, hypersalivation, retching, flatulence, tenesmus, intestinal stasis, digestive tract hypermotility, melena, pancreatitis, involuntary defecation
General: Lethargy, anorexia, weight loss, polydipsia, hyperthermia, pale mucous membrane, general pain, collapse, dehydration, edema
Dermatologic: Pruritus, dermatitis and eczema, alopecia, erythema, papilloma, bacterial skin infection, skin lesion, skin and/or appendage neoplasm, pigmentation disorder, hair change, hyperkeratosis, histiocytoma, fungal skin infection, dermal cyst(s), desquamation
Behavioral: Hyperactivity, behavioral changes, anxiety, vocalization, aggression, inappropriate urination, disorientation
Neurologic: Muscle tremor, convulsion, ataxia, paresis
Respiratory: Tachypnea, dyspnea, cough
Urologic: Polyuria, urine abnormalities (hematuria, urinary tract infection, proteinuria, glucosuria, decreased urine concentration), urinary incontinence, cystitis, renal failure, renal insufficiency
Immune: Urticaria, anaphylaxis, allergic edema
Blood and lymphatic: Lymphadenopathy, anemia, hypoalbuminemia, leukopenia
Hepatic: Elevated Liver Enzymes, hepatopathy, hepatomegaly, hepatitis
Musculoskeletal: Lameness, limb weakness, myositis
Ear and labyrinth: Otitis externa
Cardio-vascular: Tachycardia
Endocrine: Diabetes mellitus, hyperglycemia
In some cases, death/euthanasia has been reported as an outcome of the adverse events listed above.
Neoplasms have been reported in dogs taking cyclosporine, including reports of lymphoma/lymphosarcoma and mast cell tumor. It is unknown if these were preexisting or developed de novo while on cyclosporine.
Diabetes mellitus has been reported; West Highland White Terriers are the most frequently reported breed.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Dechra Veterinary Products at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
-
Clinical Pharmacology:
Cyclosporine is an immunosuppressive agent that has been shown to work via suppression of T-helper and T-suppressor cells and inhibition of interleukin-2. It does not depress hematopoesis or the function of phagocytic cells. A decrease in CD4 and CD8 cells was not seen in dogs receiving 20 mg/kg/day of cyclosporine for 56 days. Sporimune (cyclosporine capsules) USP MODIFIED is not a corticosteroid or an antihistamine.
-
Metabolism:
Cyclosporine is extensively metabolized by the cytochrome P-450 enzyme system in the liver, and to a lesser degree in the gastrointestinal tract and the kidney. The metabolism of cyclosporine can be altered by the co-administration of a variety of agents (See Precautions).
-
Effectiveness Field Study:
A multisite, placebo controlled, double masked, field study was conducted in the United States and Canada using 16 investigators. Two hundred sixty five (265) dogs aged 1-10 years, weighing 4-121 lbs received either cyclosporine capsules at 5 mg/kg/day or placebo capsules. After 30 days, placebo dogs were switched to cyclosporine capsules.
Dogs were treated with cyclosporine capsules for a total of 4 months. No additional therapy with antihistamines, corticosteroids or medicated shampoos was permitted.
Evaluations for pruritus and for skin lesions to derive a Canine Atopic Dermatitis Extent and Severity Index (CADESI) score occurred at enrollment and at monthly intervals. One hundred ninety-two (192) dogs were included in the statistical analysis of effectiveness.
At the end of the 30 day placebo controlled period, CADESI scores of dogs treated with cyclosporine capsules improved by 45% from enrollment, while CADESI scores of dogs treated with placebo worsened by 9%. Seventy-four percent (74%) of cyclosporine treated dogs showed improvement in their pruritus scores over the first 30 day period, while only 24% of the placebo treated dogs showed an improvement.
Owner and Veterinary Global Assessment in response to treatment also demonstrated statistically significant (p<0.0001) improvement. After 4 weeks of therapy, Owner and Veterinary Global Assessments showed approximately twice as much improvement in the cyclosporine treated dogs as compared to placebo treated dogs.
Improvements in pruritus accompanied by 50% or 75% improvements in CADESI scores resulted in dose reductions to every other day or twice weekly respectively. Not all dogs were able to decrease to twice weekly dosing. Some animals required upward or downward dosage adjustments during the study. Such adjustments should be expected during therapy of this disease. Dogs unable to decrease from once daily dosing after 60 days were considered dose reduction failures for the purposes of the study.
The results of dose assignments, based on the study criteria, for each 4-week dosing period, are shown in the graph.
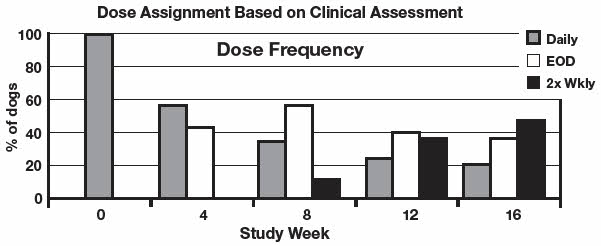
Analysis of blood levels of cyclosporine drawn during the study demonstrated no correlation between blood cyclosporine levels and CADESI scores or pruritus; therefore monitoring blood cyclosporine levels is not an appropriate predictor of effectiveness.
-
Animal Safety:
In a 52-week oral study with dose levels of 0, 1, 3, and 9 times the target initial daily dose, emesis, diarrhea and weight loss were seen in all cyclosporine treated groups with increasing frequency as the dose increased.
Multilocular papilloma-like lesions of the skin were observed in 5 out of 8 high dose animals between weeks 20 and 40. These changes regressed spontaneously after drug was withdrawn.
Other findings in the mid and high dose animals included swollen gums due to chronic gingivitis and periodontitis, lower serum albumin and higher cholesterol, triglyceride, IgA and IgG. Hematological findings consisted of anemia and decreased leukocyte counts in a few high dose animals. Erythrocyte sedimentation rates were increased at all dose levels in a dose dependent fashion. Notable histopathological findings were limited to lymphoid atrophy, hypertrophic gums (from gingivitis) and slight regenerative changes of the renal tubular epithelium in high dose animals. The findings were shown to be reversible during a 12-week recovery phase of the study.
In a 90-day study with cyclosporine, dogs were dosed in one of two patterns: either 1, 3, or 5× the maximum recommended target initial daily dose for 90 days, or 1, 3, or 5× the maximum recommended target initial daily dose for 30 days followed by tapering to mimic the recommended clinical dosing pattern. The maximum recommended dose, when administered for 90 days causes callus-like lesions on the footpads, red/swollen pinnae, mild to moderate gingival proliferation, hyperkeratotic areas on the integument, hair loss, salivation, vomiting, and diarrhea/abnormal stools. These clinical signs lessened in severity or resolved as the drug was tapered to a lower dose. Increased erythrocyte sedimentation rate, hyperproteinemia, hyperglobulinemia, hypoalbuminemia, hypocalcemia, hypophosphatemia, and hypomagnesemia were observed at three and five times the maximum recommended dose. These resolved as the dose was tapered.
When administered at higher than the maximum recommended dose, raised skin lesions, papilloma-like areas on the integument, popliteal lymph node enlargement, and weight loss were also seen. There were no cyclosporine related changes in urinalysis, ECG, blood pressure, or ophthalmologic exams.
Gross necropsy revealed epithelial changes consistent with those seen on physical examination. Proliferation of gingiva and toe pad epithelium was seen in all cyclosporine dosed groups, and was seen in a dose dependent fashion. The degree of the proliferation was greater in dogs in the non-tapered groups as compared to the tapered groups. Similar changes were noted on histopathologic examination of the cutaneous changes seen on physical examination. These lesions were characterized by epidermal hyperplasia, chronic dermatitis and hyperkeratosis.
Methylprednisolone combination: Twenty-four dogs were administered 1 mg/kg/day methylprednisolone alone for 14 days followed by 20 mg/kg/day cyclosporine either alone or in combination with methylprednisolone, or placebo for 14 days. There was no evidence of seizures/convulsions or neurological signs.
Vaccination effect: The effect of cyclosporine administration on the immunological response to vaccination was evaluated in a study in which 16 dogs were dosed with either cyclosporine at 20 mg/kg/day (4× the initial daily dose) or placebo for 56 days. All dogs were vaccinated on Day 27 with a killed commercial rabies virus and a multivalent vaccine (DHLPP) which included a modified live virus. Antibody titers for rabies, canine distemper, canine adenovirus type 2, parainfluenza, parvovirus, Leptospira canicola, and Leptospira icterohaemmorrhagiae were examined on Days 0, 27 (prior to vaccination), 42 and 56. Quantification of CD4, CD8, and CD3 T-lymphocytes was analyzed.
Clinical changes included soft stool and dermatologic changes consistent with those seen in previous studies. Antibody titers did not rise in dogs treated with cyclosporine or the placebo for any component of the multivalent vaccine which included a modified live virus while all animals demonstrated a significant increase in antibody rabies titer by Day 42 or 15 days post-revaccination. No effect was seen on T-lymphocytes.
- Storage Conditions:
-
How Supplied:
Sporimune is supplied in packages of 15 unit-dose blisters as follows:
10 mg: oval, off-white capsules imprinted with black "D 10" (NDC 17033-260-15).
25 mg: oval, gray capsules imprinted with black "D 25" (NDC 17033-261-15).
50 mg: oblong, off-white capsules imprinted with black "D 50" (NDC 17033-262-15).
100 mg: oblong, gray capsules imprinted with black "D 100" (NDC 17033-263-15). - SPL UNCLASSIFIED SECTION
-
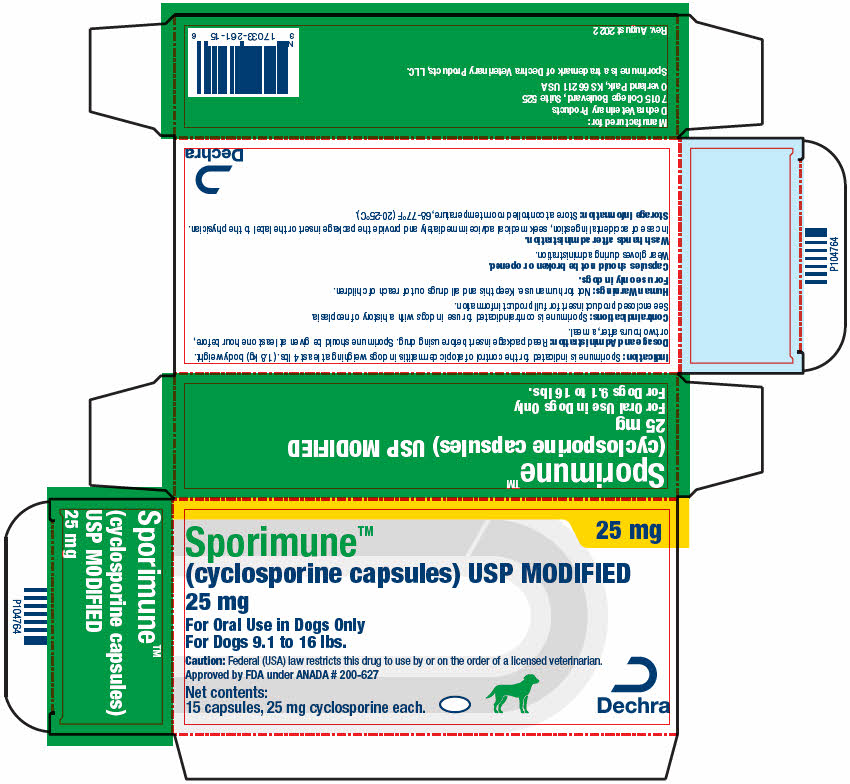
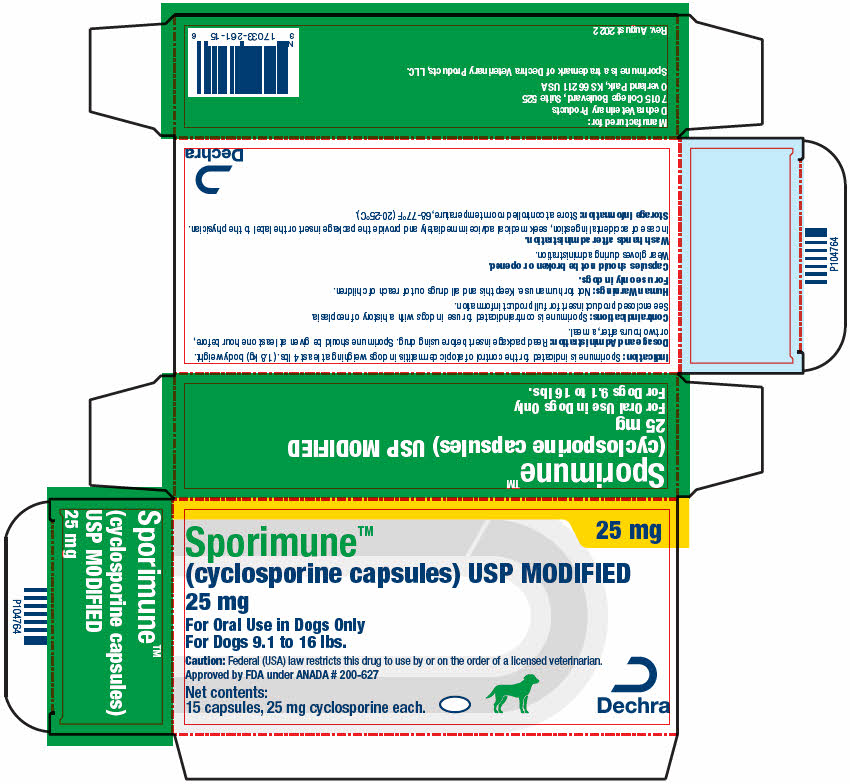
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Blister Pack Carton
25 mg
Sporimune™
(cyclosporine capsules) USP MODIFIED
25 mg
For Oral Use in Dogs Only
For Dogs 9.1 to 16 lbs.Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA # 200-627Net contents:
15 capsules, 25 mg cyclosporine each.Dechra
-
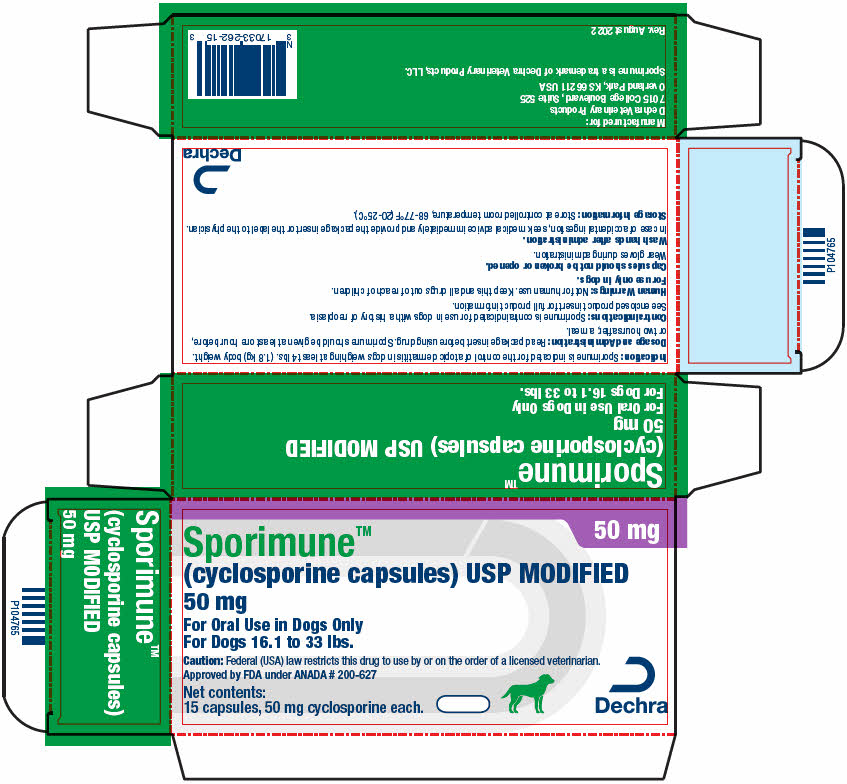
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Blister Pack Carton
50 mg
Sporimune™
(cyclosporine capsules) USP MODIFIED
50 mg
For Oral Use in Dogs Only
For Dogs 16.1 to 33 lbs.Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA # 200-627Net contents:
15 capsules, 50 mg cyclosporine each.Dechra
-
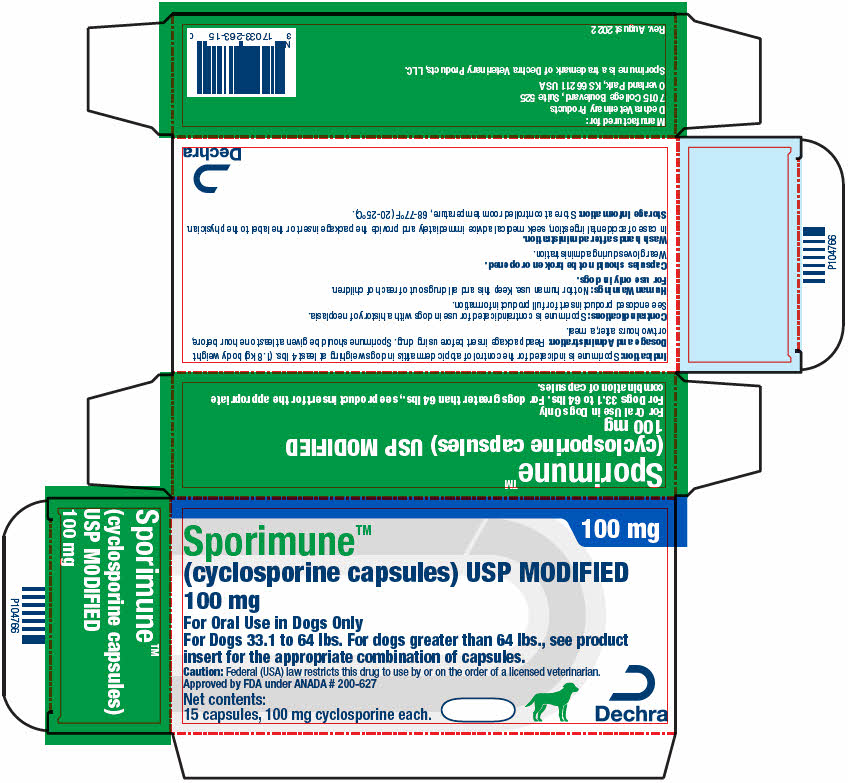
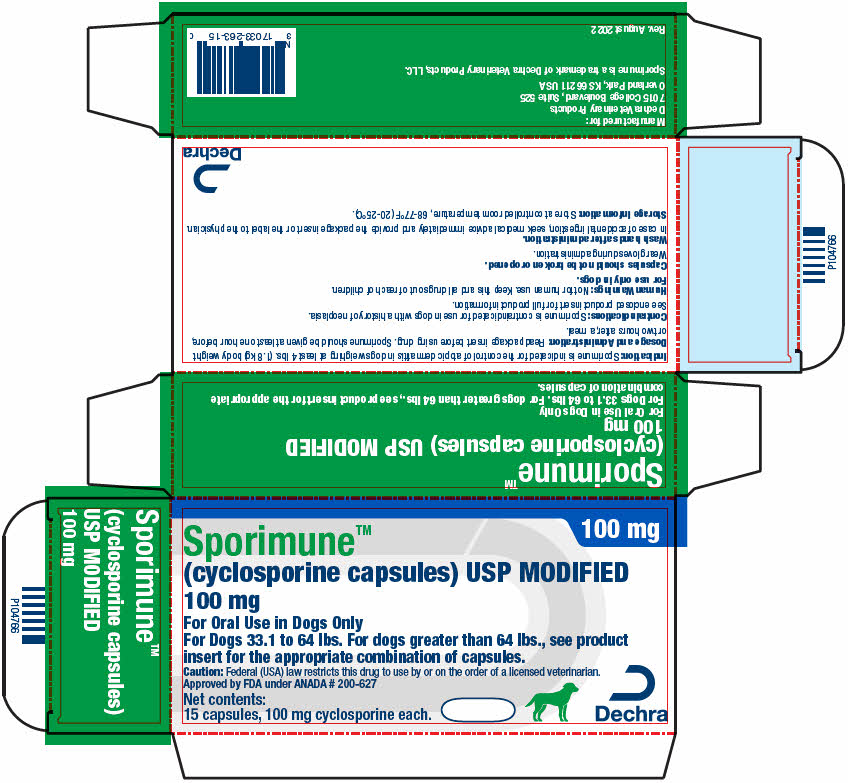
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Blister Pack Carton
100 mg
Sporimune™
(cyclosporine capsules) USP MODIFIED
100 mg
For Oral Use in Dogs Only
For Dogs 33.1 to 64 lbs. For dogs greater than 64 lbs., see product
insert for the appropriate combination of capsules.Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA # 200-627Net contents:
15 capsules, 100 mg cyclosporine each.Dechra
-
INGREDIENTS AND APPEARANCE
SPORIMUNE
cyclosporine capsuleProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-261 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (Cyclosporine - UNII:83HN0GTJ6D) CYCLOSPORINE 25 mg Product Characteristics Color GRAY Score no score Shape OVAL Size 5mm Flavor Imprint Code D;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-261-15 1 in 1 CARTON 1 15 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200627 10/02/2023 SPORIMUNE
cyclosporine capsuleProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-262 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (Cyclosporine - UNII:83HN0GTJ6D) CYCLOSPORINE 50 mg Product Characteristics Color WHITE Score no score Shape OVAL Size 10mm Flavor Imprint Code D;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-262-15 1 in 1 CARTON 1 15 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200627 10/02/2023 SPORIMUNE
cyclosporine capsuleProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-263 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (Cyclosporine - UNII:83HN0GTJ6D) CYCLOSPORINE 100 mg Product Characteristics Color GRAY Score no score Shape OVAL Size 20mm Flavor Imprint Code D;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-263-15 1 in 1 CARTON 1 15 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200627 10/02/2023 Labeler - Dechra Veterinary Products LLC (362142734)