Label: API FIN AND BODY CURE- doxycycline hyclate powder
- NDC Code(s): 17163-017-16
- Packager: MARS FISHCARE NORTH AMERICA, INC.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions for Use:
For best results, remove activated carbon or filter cartridge from filter and continue aeration.
For each 10 gallons (38 L) of water, empty one packet directly into aquarium.
Repeat dose after 24 hours. Wait another 24 hours then change 25% of the aquarium water.
Repeat this treatment for a second time, for a total of 4 doses.
Then make a final 25% water change and add fresh activated carbon or replace filter cartridge.
Treatment may be repeated, if necessary.
Note: This medication will cause a slight discoloration of water which can be removed with API BIO-CHEM ZORB or activated carbon
This package treats up to 100 gallons.
Four doses required for full course of treatment.
-
INDICATIONS & USAGE
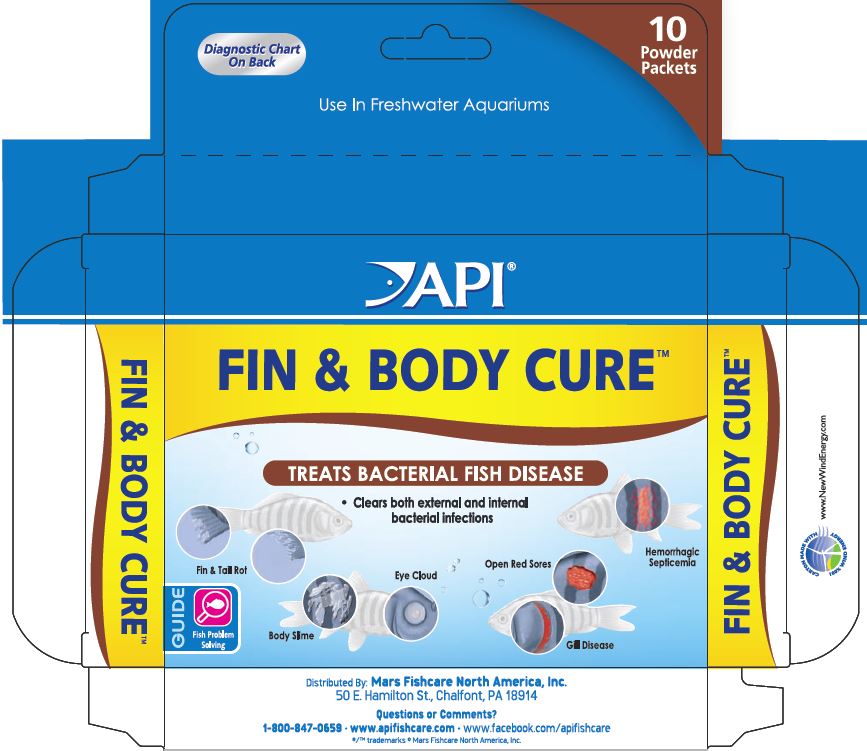
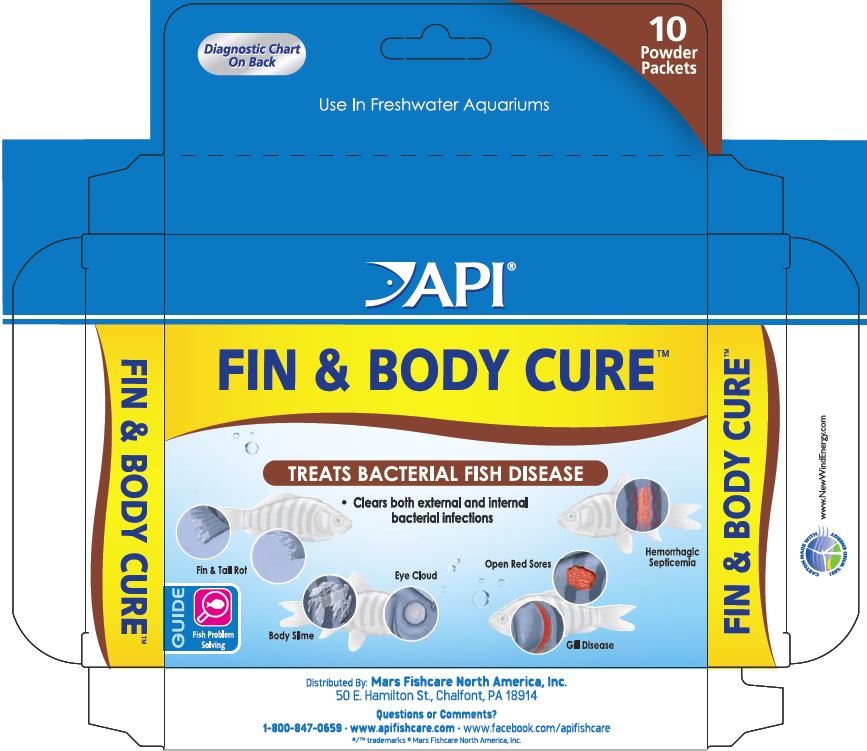
Diagnostic Chart
Fin and Tail Rot:
Fins appear ragged and split. In severe cases, fins and tail are almost completely eroded. Secondary fungal infections, observed as white, "cottony" growth, commonly occur.
Bacterial Gill Disease:
Fish may breathe heavily and show bright red gills. Fish may sit at bottom of aquarium or near the water's surface. However, visual diagnosis is often difficult.
Hemorrhagic Septicemia:
A bacterial infection of the bloodstream. Fish may show blood streaks on the body and fins, bleeding (red patches) and localized swelling.
Open Red Sores:
A bacterial infection that causes open red sores on body. Infection can range from very small ulcers to deep skin lesions. Coloring can range from reddish or gray ulcers to very dark areas associated with necrotic (dying) tissue.
Body Slime and Eye Cloud:
Eyes develop a white haze and/or protrude from the head. Slimy patches appear on the body. Fish may scratch on objects and exhibit rapid breathing. Infection may cause abmormal osmotic funstion resulting in fluid accumulation to the eyes and/or body.
-
WARNINGS AND PRECAUTIONS
KEEP OUT OF THE REACH OF CHILDREN.
For aquarium use only.
Not for human consumption or for the treatment of fish intended for human consumption.
Avoid contact with skin and eyes.
May cause irritation.
! WARNING:
This product can expose you to chemicals including Silica, crystalline, which is known to the State of California to cause cancer.
For more information go to www.P65Warnings.ca.gov.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
API FIN AND BODY CURE
doxycycline hyclate powderProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:17163-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYCYCLINE HYCLATE (UNII: 19XTS3T51U) (DOXYCYCLINE ANHYDROUS - UNII:334895S862) DOXYCYCLINE ANHYDROUS 250 mg in 2 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17163-017-16 10 in 1 BOX 1 2 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2017 Labeler - MARS FISHCARE NORTH AMERICA, INC. (049630700)