Label: HYDROCORTISONE (antipruritic- anti-itch ointment
- NDC Code(s): 68001-526-45
- Packager: BluePoint Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- in the eyes
- for diaper rash
- for external genital or feminine itching if you have a vaginal discharge
- more than the recommended daily dosage unless directed by a doctor
- this product in the rectum by using fingers or any mechanical device or applicator
Ask a doctor before use if you are using any other hydrocortisone product
-
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily. Children under 2 years of age: consult a doctor.
- When used for anal itching, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry, patting or blotting with bathroom tissue or soft cloth before applying.
- Children under 12: consult a doctor before using for anal itching.
- Other Information
- Inactive Ingredients
- Questions?
- Label
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
antipruritic (anti-itch) ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-526 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-526-45 1 in 1 CARTON 05/17/2023 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/17/2023 Labeler - BluePoint Laboratories (985523874) Establishment Name Address ID/FEI Business Operations Gopaldas Visram & Co., Ltd 858030888 manufacture(68001-526)

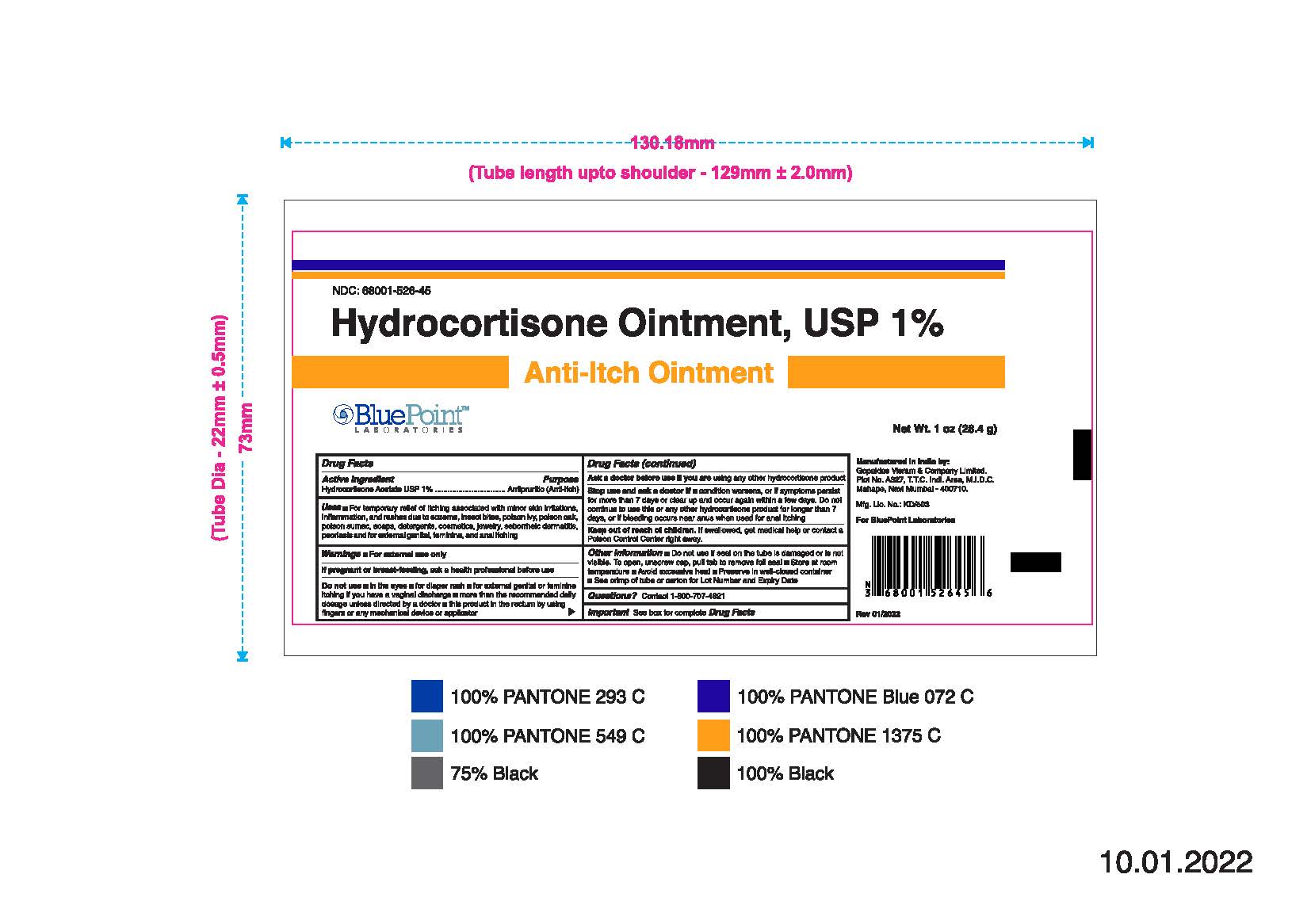
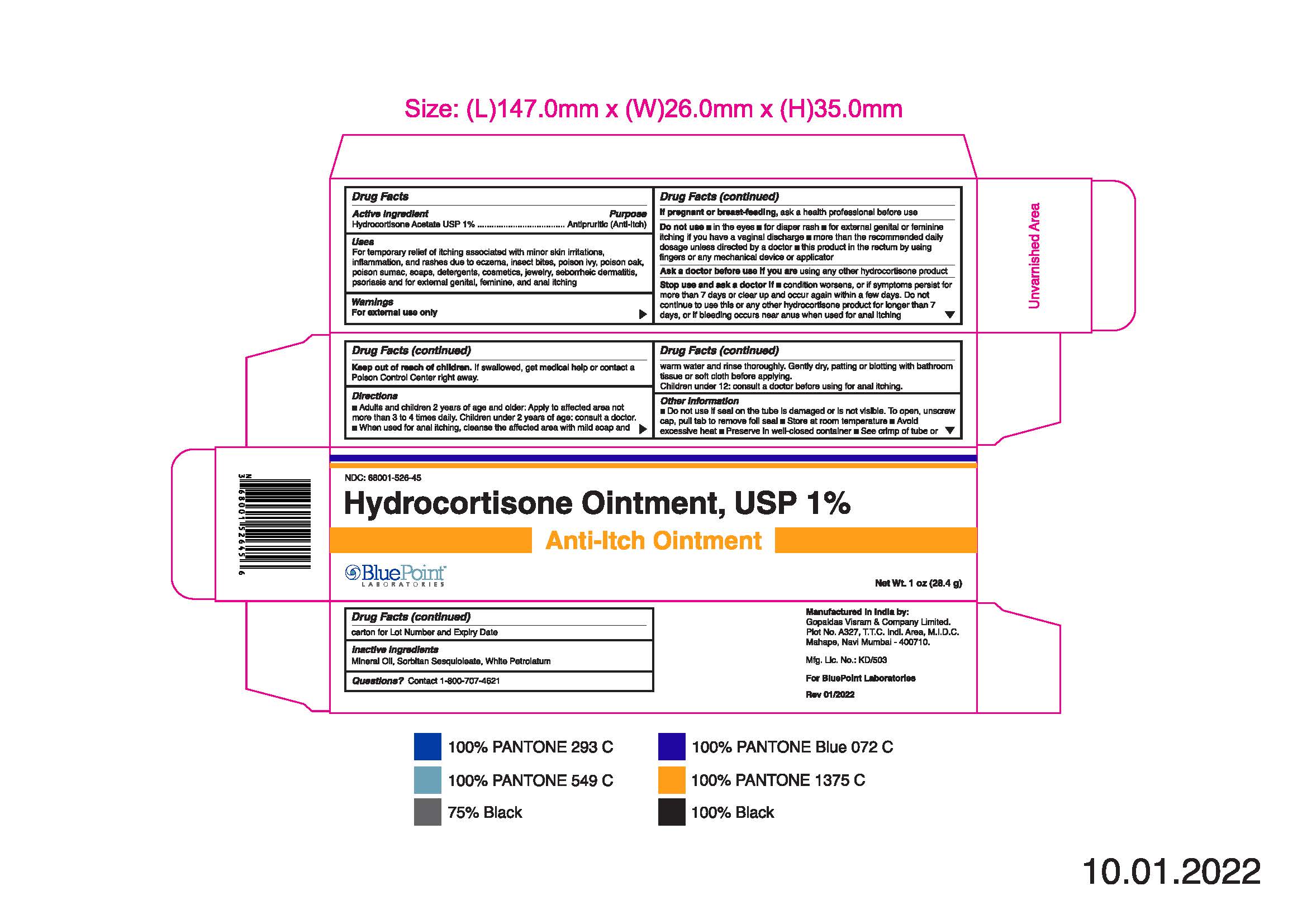
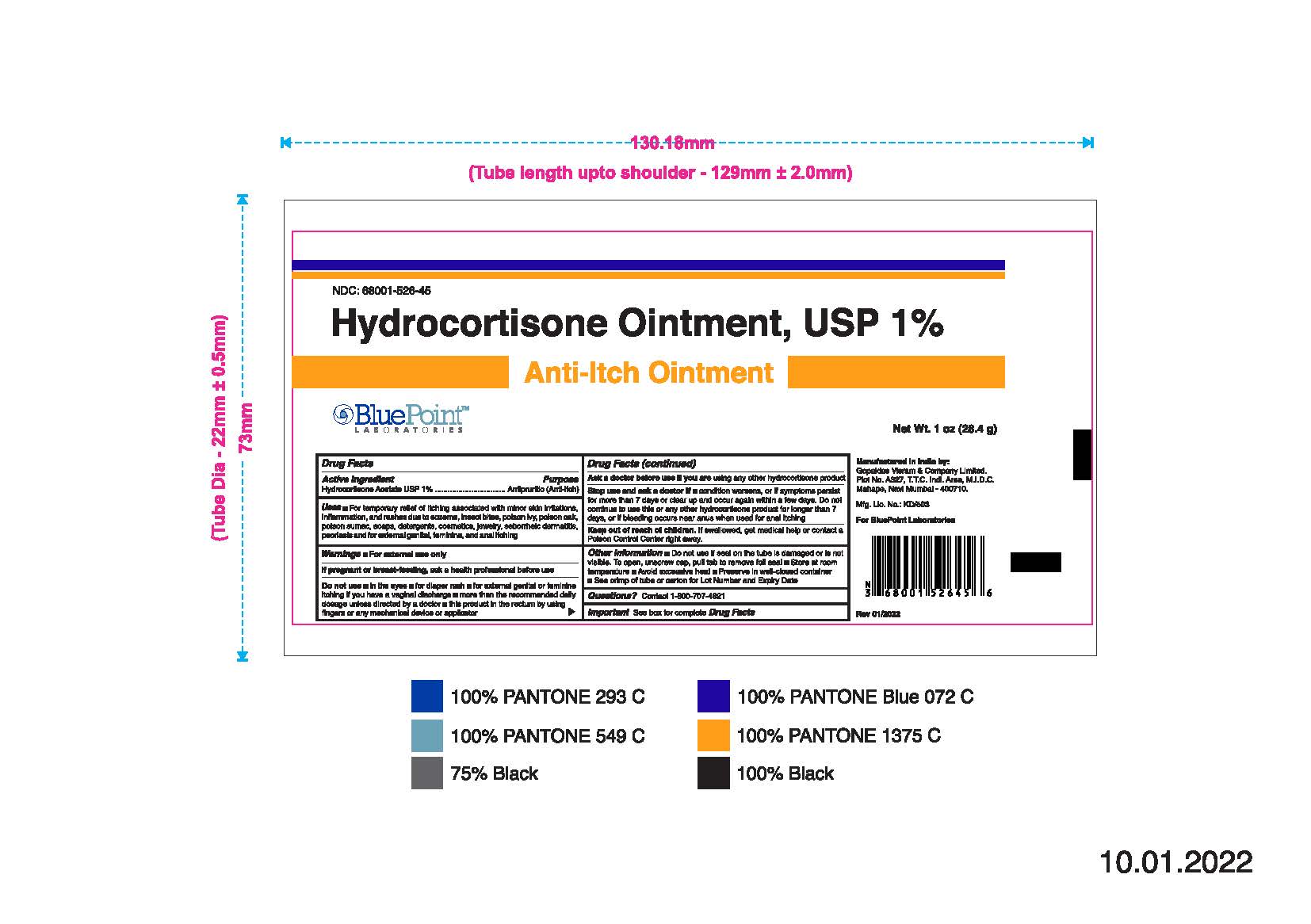
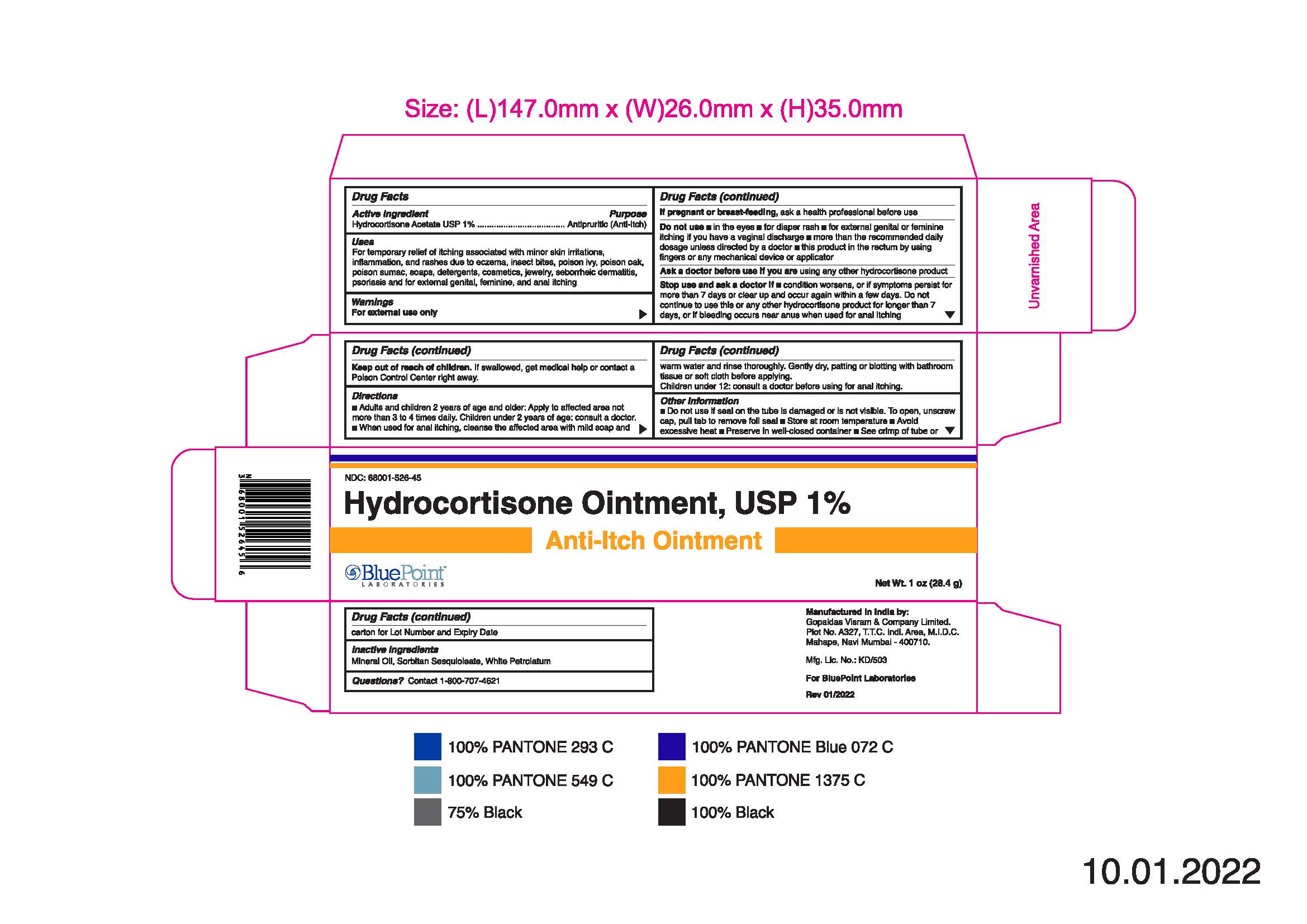 Hydrocortisone Ointment, USP 1%
Hydrocortisone Ointment, USP 1%