Label: HETASTARCH IN SODIUM CHLORIDE- hetastarch injection, solution
- NDC Code(s): 0409-7248-03, 0409-7248-13
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use 6% Hetastarch in 0.9% Sodium Chloride Injection safely and effectively. See full prescribing information for 6% Hetastarch in 0.9% Sodium Chloride Injection.
6% Hetastarch in 0.9% Sodium Chloride Injection, for intravenous use
Initial U.S. Approval: 1991WARNING: MORTALITY;
KIDNEY INJURY; COAGULOPATHYSee full prescribing information for complete boxed warning.
- •
- Use of hydroxyethyl starch (HES) products, including 6% Hetastarch in 0.9% Sodium Chloride Injection, increases risk of
- •
- DO NOT use HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection, unless adequate alternative treatment is unavailable. (1)
INDICATIONS AND USAGE
- •
- 6% Hetastarch in 0.9% Sodium Chloride Injection is a hetastarch indicated for treatment of hypovolemia when plasma volume expansion is desired in settings where adequate alternative treatment is unavailable. (1)
- •
- 6% Hetastarch in 0.9% Sodium Chloride Injection in leukapheresis has shown to be safe and efficacious in improving the harvesting and increasing the yield of granulocytes by centrifugal means. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- •
- 30 g hetastarch in 500 mL 0.9% sodium chloride injection. (3)
CONTRAINDICATIONS
- •
- Do not use HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection, unless adequate alternative treatment is unavailable.
WARNINGS AND PRECAUTIONS
- •
- Avoid use in patients with pre-existing renal dysfunction (5.1)
- •
- Increased risk of mortality and acute kidney injury (AKI) in critically ill patients, including patients with sepsis; surgical patients; and blunt trauma patients (5.1)
- •
- Discontinue use of 6% Hetastarch in 0.9% Sodium Chloride Injection at the first sign of renal injury (5.1)
- •
- Continue to monitor renal function for at least 90 days as use of RRT has been reported up to 90 days after administration of HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection (5.1)
- •
- 6% Hetastarch in 0.9% Sodium Chloride Injection is not recommended for use as a cardiac bypass pump prime, while the patient is on cardiopulmonary bypass, or in the immediate period after the pump has been discontinued because of the risk of increasing coagulation abnormalities and bleeding in patients whose coagulation status is already impaired. Discontinue use of 6% Hetastarch in 0.9% Sodium Chloride Injection at first sign of coagulopathy (5.2)
- •
- Monitor liver function in patients receiving HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection (5.2)
ADVERSE REACTIONS
- •
- Most common adverse reactions are hypersensitivity, coagulopathy, hemodilution, circulatory overload and metabolic acidosis. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100 or electronically at ProductComplaintsPP@hospira.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Revised: 7/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MORTALITY; KIDNEY INJURY; COAGULOPATHY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults
2.2 Leukapheresis
2.3 Direction for use for 6% Hetastarch in 0.9% Sodium Chloride Injection
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Mortality and Renal Dysfunction
5.2 Coagulopathy
5.3 Hypersensitivity Reactions
5.4 Circulatory Overload
5.5 Liver Function Test
5.6 Drug/Laboratory Test Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MORTALITY; KIDNEY INJURY; COAGULOPATHY
- •
-
Use of hydroxyethyl starch (HES) products, including 6% Hetastarch in 0.9% Sodium Chloride Injection, increases risk of
- o
- Mortality
- o
- Kidney injury (5.1)
- o
- Coagulopathy (5.2)
- •
- DO NOT use HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection, unless adequate alternative treatment is unavailable. (1)
-
1 INDICATIONS AND USAGE
6% Hetastarch in 0.9% Sodium Chloride Injection is indicated in the treatment of hypovolemia when plasma volume expansion is desired in settings where adequate alternative treatment is unavailable. It is not a substitute for blood or plasma.
The adjunctive use of 6% Hetastarch in 0.9% Sodium Chloride Injection in leukapheresis has also been shown to be safe and efficacious in improving the harvesting and increasing the yield of granulocytes by centrifugal means.
-
2 DOSAGE AND ADMINISTRATION
Dosage for Acute Use in Plasma Volume Expansion
6% Hetastarch in 0.9% Sodium Chloride Injection is administered by intravenous infusion only. Total dosage and rate of infusion depend upon the amount of blood or plasma lost and the resultant hemoconcentration.
2.1 Adults
The amount usually administered is 500 to 1000 mL. Doses of more than 1500 mL per day for the typical 70 kg patient (approximately 20 mL per kg of body weight) are usually not required. Higher doses have been reported in postoperative and trauma patients where severe blood loss has occurred [see Warnings and Precautions (5)].
2.2 Leukapheresis
250 to 700 mL of 6% Hetastarch in 0.9% Sodium Chloride Injection with citrate anticoagulant is administered by aseptic addition to the input line of the centrifugation apparatus at a ratio of 1:8 to 1:13 to venous whole blood. The 6% Hetastarch in 0.9% Sodium Chloride Injection and citrate should be thoroughly mixed to assure effective anticoagulation of blood as it flows through the leukapheresis machine.
2.3 Direction for use for 6% Hetastarch in 0.9% Sodium Chloride Injection
- •
- Do not use plastic container in series connection. If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use only if solution is clear and container and seals are intact.
- •
- Intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
- •
- Withdraw or expel all air from the bag through the medication port prior to infusion if administration is by pressure infusion.
- •
- For single use only. The solution contains no bacteriostat, antimicrobial agent or added buffers (except for pH adjustment) and is intended only for single-dose injection. When smaller doses are required the unused portion should be discarded.
CAUTION: Before administering to the patient, review these directions:
Visual Inspection
- •
- Do not remove the plastic infusion container from its overwrap until immediately before use.
- •
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- •
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter.
- •
- Any container which is suspect should not be used.
To Open
- •
- Tear overwrap down at notch and remove solution container.
- •
- Check for minute leaks by squeezing solution container firmly.
- •
- If any leaks are found, discard solution as sterility may be impaired.
Preparation for Administration
- •
- Remove plastic protector from sterile set port at bottom of container.
- •
- Attach administration set. Refer to complete directions accompanying set.
When stored at room temperature, 6% Hetastarch in 0.9% Sodium Chloride Injection admixtures of 500–560 mL with citrate concentrations up to 2.5% were compatible for 24 hours. The safety and compatibility of additives other than citrate have not been established.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Mortality and Renal Dysfunction
- •
- Critically ill patients, including patients with sepsis, are at increased risk of mortality and acute kidney injury (AKI), including need for renal replacement therapy (RRT)
- •
- Surgery patients are at increased risk of mortality and AKI
- •
- Blunt trauma patients are at risk of mortality and AKI
- •
- Avoid use in patients with pre-existing renal dysfunction
- •
- Discontinue use of 6% Hetastarch in 0.9% Sodium Chloride Injection at the first sign of renal injury
- •
- Continue to monitor renal function for at least 90 days as use of RRT has been reported up to 90 days after administration of HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection
5.2 Coagulopathy
- •
- 6% Hetastarch in 0.9% Sodium Chloride Injection is not recommended for use as a cardiac bypass pump prime, while the patient is on cardiopulmonary bypass, or in the immediate period after the pump has been discontinued because of the risk of increasing coagulation abnormalities and bleeding in patients whose coagulation status is already impaired. Monitor the coagulation status of surgery patients, as excess bleeding has been reported with HES solutions in this population. Discontinue use of 6% Hetastarch in 0.9% Sodium Chloride Injection at first sign of coagulopathy1–2
6% Hetastarch in 0.9% Sodium Chloride Injection has not been adequately evaluated to establish its safety in uses over extended periods other than leukapheresis. 6% Hetastarch in 0.9% Sodium Chloride Injection has been associated with coagulation abnormalities in conjunction with an acquired, reversible von Willebrand's-like syndrome and/or Factor VIII deficiency when used over a period of days. Replacement therapy should be considered if a severe Factor VIII deficiency is identified. If a coagulopathy develops, it may take several days to resolve. Certain conditions may affect the safe use of 6% Hetastarch in 0.9% Sodium Chloride Injection on a chronic basis. For example, in patients with subarachnoid hemorrhage where 6% Hetastarch in 0.9% Sodium Chloride Injection is used repeatedly over a period of days for the prevention of cerebral vasospasm, significant clinical bleeding may occur. Intracranial bleeding resulting in death has been reported.3
Slight declines in platelet counts and hemoglobin levels have been observed in donors undergoing repeated leukapheresis procedures using 6% Hetastarch in 0.9% Sodium Chloride Injection due to the volume expanding effects of hetastarch and to the collection of platelets and erythrocytes. Hemoglobin levels usually return to normal within 24 hours. Hemodilution by 6% Hetastarch in 0.9% Sodium Chloride Injection may also result in 24 hour declines of total protein, albumin, calcium, and fibrinogen levels. Regular and frequent clinical evaluation and complete blood counts (CBC) are necessary for proper monitoring of 6% Hetastarch in 0.9% Sodium Chloride Injection use during leukapheresis. If the frequency of leukapheresis is to exceed the guidelines for whole blood donation, you may wish to consider the following additional tests: total leukocyte and platelet counts, leukocyte differential count, hemoglobin and hematocrit, prothrombin time (PT), and partial thromboplastin time (PTT).
5.3 Hypersensitivity Reactions
Life threatening anaphylactic/anaphylactoid reactions including death have been rarely reported with 6% Hetastarch in 0.9% Sodium Chloride Injection. Patients may develop hypersensitivity reaction to corn starch from which this product is made. If a hypersensitivity reaction occurs, administration of the drug should be discontinued immediately and the appropriate treatment and supportive measures should be undertaken until symptoms have resolved.
5.4 Circulatory Overload
6% Hetastarch in 0.9% Sodium Chloride Injection has not been adequately evaluated to establish its safety in situations other than treatment of hypovolemia in elective surgery.
Large volumes of 6% Hetastarch in 0.9% Sodium Chloride Injection may transiently alter the coagulation mechanism due to hemodilution and a direct inhibitory action on Factor VIII. Administration of volumes of 6% Hetastarch in 0.9% Sodium Chloride Injection that are greater than 25% of the blood volume in less than 24 hours may cause significant hemodilution reflected by lower hematocrit and plasma protein values. Administration of packed red cells, platelets, or fresh frozen plasma should be considered if clinically indicated.
When using 6% Hetastarch in 0.9% Sodium Chloride Injection for plasma volume expansion, caution should be taken to avoid excessive hemodilution and circulatory overload especially in those patients at risk for developing congestive heart failure and pulmonary edema. 6% Hetastarch in 0.9% Sodium Chloride Injection is primarily excreted via the kidneys so caution should be exercised in patients who have impaired renal function. Although the risk of circulatory overload is largely dependent on the clinical circumstances, use of doses higher than 20 mL/kg/24h will increase the risk significantly. Increased risk of coagulation abnormalities and bleeding is also associated with higher doses. Monitor patients' vital signs and hemoglobin, hematocrit, platelet count, prothrombin time and partial thromboplastin time.
5.5 Liver Function Test
- •
- Monitor liver function in patients receiving HES products, including 6% Hetastarch in 0.9% Sodium Chloride Injection
5.6 Drug/Laboratory Test Interactions
Bilirubin Levels
Indirect bilirubin levels of 8.3 mg/L (normal 0.0-7.0 mg/L) have been reported in 2 out of 20 normal subjects who received multiple infusions of 6% Hetastarch in 0.9% Sodium Chloride Injection. Total bilirubin was within normal limits at all times; indirect bilirubin returned to normal by 96 hours following the final infusion. The significance, if any, of these elevations is not known; however, caution should be observed before administering 6% Hetastarch in 0.9% Sodium Chloride Injection to patients with a history of liver disease.
Serum Amylase Levels
Elevated serum amylase levels may be observed temporarily following administration of 6% Hetastarch in 0.9% Sodium Chloride Injection although no association with pancreatitis has been demonstrated. Serum amylase levels cannot be used to assess or to evaluate for pancreatitis for 3-5 days after administration of 6% Hetastarch in 0.9% Sodium Chloride Injection. Elevated serum amylase levels persist for longer periods of time in patients with renal impairment. Hetastarch has not been shown to increase serum lipase.
Hemodialysis
6% Hetastarch in 0.9% Sodium Chloride Injection is not eliminated by hemodialysis. The utility of other extracorporeal elimination techniques has not been evaluated.
-
6 ADVERSE REACTIONS
Serious adverse reactions reported in postmarket clinical trials include increased mortality and AKI (including need for RRT) in critically ill subjects, including subjects with sepsis, and surgical subjects. Clinical trials have also shown increased mortality and AKI in blunt trauma subjects. Increased coagulopathy was reported in surgical subjects.
Most common adverse reactions are hypersensitivity, coagulopathy, hemodilution, circulatory overload and metabolic acidosis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
(Note: All of the studies listed below used licensed HES products except for reference 4.)
A randomized controlled trial (N=804) in severe sepsis patients using HES product (not approved in the U.S.) reported increased mortality (relative risk, 1.17; 95% CI, 1.01 to 1.36; p=0.03) and RRT (relative risk, 1.35; 95% CI, 1.01 to 1.80; p=0.04) in the HES treatment arm.4
Another randomized controlled trial (N=196) using different HES in severe sepsis patients reported no difference in mortality (relative risk, 1.20; 95% CI, 0.83 to 1.74; p=0.33) and a trend for RRT (relative risk, 1.83; 95% CI, 0.93 to 3.59; p=0.06) in HES patients.5
A randomized controlled trial (N=7000) using different HES in a heterogeneous patient population consisting of critically ill adult patients admitted to the ICU reported no difference in mortality (relative risk, 1.06; 95% CI, 0.96 to 1.18; p=0.26) but increased use of RRT (relative risk, 1.21; 95% CI, 1.00 to 1.45; p=0.04) in HES patients.6
In a retrospective study of adult patients (N=1442) undergoing pulmonary or esophageal surgery who were prophylactically fluid restricted during the procedure, 74 developed AKI (5.1%) within the first 72 hours postoperatively. Fluid restriction neither increased nor was a risk factor for AKI. AKI occurred more often when HES products were administered to patients with decreased renal function or having >2 risk factors with normal renal function, whereas restriction of crystalloid was unrelated to AKI, regardless of preoperative renal function.12
In a retrospective case series of high-risk adult vascular surgery patients (N=796) receiving fluid therapy during a vascular surgery procedure, logistic regression analysis using prespecified confounding variables or suspected risk factors for AKI showed that intraoperative administration of an HES product was associated with increased likelihood of 30-day mortality and need for RRT, compared with use of crystalloids alone.13
In a retrospective study of adult subjects undergoing elective noncardiac surgery, patients (N=14,680) receiving an HES product and crystalloid were propensity-matched with patients (N=14,680) receiving only crystalloid. After controlling for potential confounding variables, odds of experiencing AKI of severe intensity with HES was 21% greater than with crystalloid alone. In addition, AKI risk increased as a function of HES volume.14
In a prospective observational study assessing the impact of HES products on recipient renal graft outcomes in brain-dead organ donors, data were obtained on 986 kidneys transplanted from 529 donors. Kidneys from donors who received HES had a higher rate of delayed graft function in recipient subjects (41% versus 31%). After accounting for the propensity of donors to receive HES products, HES product administration was independently associated with an increased risk of delayed graft function in recipients. A dose response relationship also was evident.15
In a randomized, controlled trial of adult subjects (N=33) undergoing elective cystectomy comparing an HES product versus lactated Ringer's, administration of HES reduced clot strength (Maximum Amplitude; p<0.001) and increased blinded evaluation of perioperative blood loss by more than 50% (2181 mL versus 1370 mL, respectively; p=0.04). There was no significant between-group difference with respect to frequency of reoperation or length of hospital stay.16
In a prospective, sequential, observational study in adult subjects undergoing open heart surgery in association with cardiopulmonary bypass, fluid therapy using only an HES product (2004–2006, N=2137), 4% gelatin (2006–2008, N=2324) and crystalloids (N=2017, 2008–2010) led to increased need for renal replacement therapy after HES and gelatin, compared with crystalloid. Propensity score stratification confirmed greater use of RRT in the HES and gelatin periods compared to the crystalloid period. Fluid intake was higher in the crystalloid group only during the first 20 hours.17
In a retrospective observational study, 606 adult patients underwent open heart surgery in association with cardiopulmonary bypass. Until July 2013 they received an HES product (N=247) both as pump prime (1500 mL) and intraoperative fluid replacement (1000 mL), but only crystalloid (N=359) from August 2013 onward. The frequency (percent) of postoperative AKI was higher in patients receiving HES (N=53; 21.5%) than those receiving crystalloid (N=34; 9.5%). Surgical revision for rebleeding also was higher for HES (N=11; 4.6%) than for crystalloid (N=5; 1.4%).18
In a meta-analysis of RCTs (n=15) in adult subjects (N=4409) undergoing surgery who received an HES product, significantly more HES subjects (83/2157; 3.8%) than controls (56/2252; 2.5%) underwent RRT (relative risk, 1.44; 95% CI, 1.04, 2.01).19
In a retrospective observational study of adult blunt and penetrating trauma patients, use of an HES product was a significant independent predictor of AKI after blunt trauma, but not penetrating trauma, in multiple logistic regression analysis. In separate logistic regression models, HES also was a significant predictor of mortality after blunt trauma but not penetrating trauma.20
In a retrospective observational study of severely injured adult blunt (89%) and penetrating (11%) trauma patients (N=413) admitted to the ICU, 103 patients developed AKI within the first week of ICU admission. AKI was associated with increased 30-day (17.5% versus 5.8%, AKI versus non-AKI cohorts, respectively) and 1-year mortality (26.2% versus 7.1%). Univariate and multivariable regression analyses of prespecified risk factors for AKI found that volume loading using an HES product was independently associated with postinjury AKI within the first 24 hours.21
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily post-approval from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
The following adverse reactions have been identified and reported during the post-approval use of HES products:
Hypersensitivity reactions
including death, life-threatening anaphylactic/anaphylactoid reactions, cardiac arrest, ventricular fibrillation, severe hypotension, non-cardiac pulmonary edema, laryngeal edema, bronchospasm, angioedema, wheezing, restlessness, tachypnea, stridor, fever, chest pain, bradycardia, tachycardia, shortness of breath, chills, urticaria, pruritus, facial and periorbital edema, coughing, sneezing, flushing, erythema multiforme, and rash [see Warnings and Precautions (5.3)].
Cardiovascular reactions
including circulatory overload, congestive heart failure, and pulmonary edema [see Warnings and Precautions (5.4)].
Hematologic reactions
including intracranial bleeding, bleeding and/or anemia due to hemodilution [see Warnings and Precautions (5.4)] and/or Factor VIII deficiency, acquired von Willebrand's-like syndrome, and coagulopathy including rare cases of disseminated intravascular coagulopathy and hemolysis.
Metabolic reactions
including metabolic acidosis.
Other reactions
including vomiting, peripheral edema of the lower extremities, submaxillary and parotid glandular enlargement, mild influenza-like symptoms, headaches, and muscle pains. Hydroxyethyl starch-associated pruritus has been reported in some patients with deposits of hydroxyethyl starch in peripheral nerves.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Hetastarch has been shown to have an embryocidal effect on New Zealand rabbits when given intravenously over the entire organogenesis period in a daily dose 1/2 times the maximum recommended therapeutic human dose (1500 mL) and on BD rats when given intraperitoneally, from the 16th to the 21st day of pregnancy, in a daily dose 2.3 times the maximum recommended therapeutic human dose. When hetastarch was administered to New Zealand rabbits, BD rats, and swiss mice with intravenous daily doses of 2 times, 1/3 times, and 1 times the maximum recommended therapeutic human dose respectively over several days during the period of gestation, no evidence of teratogenicity was evident.
There are no adequate and well-controlled studies in pregnant women. 6% Hetastarch in 0.9% Sodium Chloride Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
11 DESCRIPTION
6% Hetastarch in 0.9% Sodium Chloride Injection is a sterile, nonpyrogenic solution for intravenous administration.
Each 100 mL contains:
Hetastarch............................................................................ 6 g
Sodium Chloride, USP........................................................ 0.9 g
Water for Injection, USP..................................................... qs
pH adjusted with Sodium Hydroxide, NF if necessary
Concentration of Electrolytes (mEq/L): Sodium (Na+) 154, Chloride (Cl-) 154 (not including ions for pH adjustment).
pH: 5.5 (3.5 to 7.0)
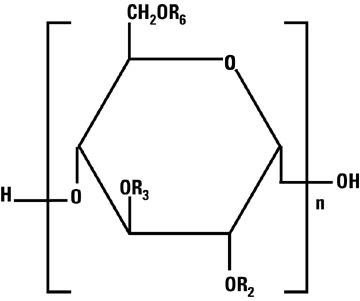
Total osmolar concentration is 308 mOsmol/liter (calc).Hetastarch is a synthetic colloid derived from a waxy starch composed almost entirely of amylopectin. Hydroxyethyl ether groups are introduced into the glucose units of the starch, and the resultant material is hydrolyzed to yield a product with a molecular weight suitable for use as a plasma volume expander and erythrocyte sedimenting agent. The molar substitution is approximately 0.75 which means hetastarch has an average of approximately 75 hydroxyethyl groups for every 100 glucose units. The weight average molecular weight is approximately 670,000 with a range of 550,000 to 800,000 and with at least 80% of the polymers falling within the range of 20,000 to 2,500,000. Hydroxyethyl groups are attached by ether linkage primarily at C-2 of the glucose unit and to a lesser extent at C-3 and C-6. The polymer resembles glycogen, and the polymerized D-glucose units are joined primarily by α-1,4 linkages with occasional α-1,6 branching linkages. The degree of branching is approximately 1:20 which means that there is one 1–6 branch for every 20 glucose monomer units.
The chemical name for hetastarch is hydroxyethyl starch.
The structural formula is as follows:
Amylopectin derivative in which R2, R3, and R6 are H or CH2CH2OH, or R6 is a branching point in the starch polymer connected through a 1-6 linkage to additional alpha-D-glucopyranosyl units.
Hetastarch is an artificial colloid pharmacologically classified as a plasma volume expander; 0.9% Sodium Chloride Injection is a fluid and electrolyte replenisher.
6% Hetastarch in 0.9% Sodium Chloride Injection is a clear, pale yellow to amber solution. Exposure to prolonged adverse storage conditions may result in a change to a turbid deep brown or the formation of a crystalline precipitate. Do not use the solution if these conditions are evident.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly.
Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The plasma volume expansion produced by 6% Hetastarch in 0.9% Sodium Chloride Injection approximates that of 5% Albumin (Human). Intravenous infusion of 6% Hetastarch in 0.9% Sodium Chloride Injection results in expansion of plasma volume.
12.2 Pharmacodynamics
6% Hetastarch in 0.9% Sodium Chloride Injection results in expansion of plasma volume that decreases over the succeeding 24 to 36 hours. The degree of plasma volume expansion and improvement in hemodynamic state depend upon the patient's intravascular status.
12.3 Pharmacokinetics
Hetastarch molecules below 50,000 molecular weight are rapidly eliminated by renal excretion. A single dose of approximately 500 mL of 6% Hetastarch in 0.9% Sodium Chloride Injection (approximately 30 g) results in elimination in the urine of approximately 33% of the dose within 24 hours. This is a variable process but generally results in an intravascular hetastarch concentration of less than 10% of the total dose injected by two weeks. A study of the biliary excretion of 6% Hetastarch in 0.9% Sodium Chloride Injection in 10 healthy males accounted for less than 1% of the dose over a 14 day period. The hydroxyethyl group is not cleaved by the body but remains intact and attached to glucose units when excreted. Significant quantities of glucose are not produced as hydroxyethylation prevents complete metabolism of the smaller polymers.
The addition of hetastarch to whole blood increases the erythrocyte sedimentation rate. Therefore, 6% Hetastarch in 0.9% Sodium Chloride Injection is used to improve the efficiency of granulocyte collection by centrifugal means.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Surgical Patients Comparative Studies
In randomized, controlled, comparative studies of 6% Hetastarch in 0.9% Sodium Chloride Injection (n=92) and Albumin (n=85) in surgical patients, no patient in either treatment group had a bleeding complication and no significant difference was found in the amount of blood loss between the treatment groups.7-10
Pediatric Postoperative Volume Expander Study
In one small double-blind study, 47 infants, children, and adolescents (ages 1 year to 15.5 years) scheduled for repair of congenital heart disease with moderate hypothermia were randomized to receive either 6% Hetastarch in 0.9% Sodium Chloride Injection or Albumin as a postoperative volume expander during the first 24 hours after surgery. Thirty-eight children required colloid replacement therapy, of which 20 children received 6% Hetastarch in 0.9% Sodium Chloride Injection. No differences were found in the coagulation parameters or in the amount of replacement fluids required in the children receiving 20 mL/kg or less of either colloid replacement therapy. In children who received greater than 20 mL/kg of 6% Hetastarch in 0.9% Sodium Chloride Injection, an increase in prothrombin time was demonstrated (p=0.006).11 There were no neonates included in this study [see Use in Specific Populations (8.4)].
Adult Critically Ill Studies
Three randomized controlled trials (RCTs) followed critically ill adult patients treated with different HES products for 90 days.
One trial (N=804) in severe sepsis patients using HES product (not approved in the U.S.) reported increased mortality (relative risk, 1.17; 95% CI, 1.01 to 1.36; p=0.03) and RRT (relative risk, 1.35; 95% CI, 1.01 to 1.80; p=0.04) in the HES treatment arm.4
Another trial (N=196) using different HES in severe sepsis patients reported no difference in mortality (relative risk,1.20; 95% CI, 0.83 to 1.74; p=0.33) and a trend for RRT (relative risk, 1.83; 95% CI, 0.93 to 3.59; p=0.06) in HES patients.5
A third trial (N=7000) using different HES in a heterogeneous patient population consisting of critically ill adult patients admitted to the ICU reported no difference in mortality (relative risk, 1.06; 95% CI, 0.96 to 1.18; p=0.26) but increased use of RRT (relative risk, 1.21; 95% CI, 1.00 to 1.45; p=0.04) in HES patients.6
-
15 REFERENCES
- 1.
- Knutson JE., et al., Does Intraoperative Hetastarch Administration Increase Blood Loss and Transfusion Requirements After Cardiac Surgery? Anesthesia Analg., 2000;90:801–7.
- 2.
- Cope JT., et al., Intraoperative Hetastarch Infusion Impairs Hemostasis After Cardiac Operations. The Annals of Thoracic Surgery, 1997;63:78–83.
- 3.
- Damon L., Intracranial Bleeding During Treatment with Hydroxyethyl Starch. New England Journal of Medicine, 1987;317(15):964–965.
- 4.
- Perner A, et al., Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis patients. The New England Journal of Medicine, 2012 July 12;367(2):124–34.
- 5.
- Guidet B, et al., Assessment of hemodynamic efficacy and safety of 6% hydroxyethyl starch 130/0.4 vs 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS Study. Critical Care, 2012 May 24;16(3):R94.
- 6.
- Myburgh JA, et al., Hydroxyethyl starch or saline for fluid resuscitation in intensive care. The New England Journal of Medicine, 2012 November 15;367(20):1901–11.
- 7.
- Diehl J., et al., Clinical Comparison of Hetastarch and Albumin in Postoperative Cardiac Patients. The Annals of Thoracic Surgery, 1982;34(6):674–679.
- 8.
- Gold M., et al., Comparison of Hetastarch to Albumin for Perioperative Bleeding in Patients Undergoing Abdominal Aortic Aneurysm Surgery. Annals of Surgery, 1990;211(4):482–485.
- 9.
- Kirklin J., et al., Hydroxyethyl Starch versus Albumin for Colloid Infusion Following Cardiopulmonary Bypass in Patients Undergoing Myocardial Revascularization. The Annals of Thoracic Surgery, 1984;37(1):40–46.
- 10.
- Moggio RA., et al., Hemodynamic Comparison of Albumin and Hydroxyethyl Starch in Postoperative Cardiac Surgery Patients. Critical Care Medicine, 1983;11(12):943–945.
- 11.
- Brutocao D., et al., Comparison of Hetastarch with Albumin for Postoperative Volume Expansion in Children After Cardiopulmonary Bypass. Journal of Cardiothoracic and Vascular Anesthesia, 1996;10(3):348–351.
- 12.
- Ahn HJ, Kim JA, Lee AR, et al. The risk of acute kidney injury from fluid restriction and hydroxyethyl starch in thoracic surgery. Anesth Analg 2016; 122(1):186–193.
- 13.
- Green RS, Butler MB, Hicks SD, et al. Effect of hydroxyethyl starch on outcomes in high-risk vascular surgery patients: A retrospective analysis. J Cardiothorac Vasc Anesth 2016; 30(4):967–72.
- 14.
- Kashy BK, Podolyak A, Makarova N, et al. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 2014; 121 (4):730–9.
- 15.
- Patel MS, Niemann CU, Sally MB, et al. The impact of hydroxyethyl starch use in deceased organ donors on the development of delayed graft function in kidney transplant recipients: A Propensity-Adjusted Analysis. Am J of Transplant 2015; 15 (8):2152–8.
- 16.
- Rasmussen KC, Johansson PI, Højskov M, et al. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg 2014; 259 (2):249–54.
- 17.
- Bayer O, Schwarzkopf D, Doenst T, et al. Perioperative fluid therapy with tetrastarch and gelatin in cardiac surgery — a prospective sequential analysis. Crit Care Med 2013; 41(11):2532–42.
- 18.
- Lagny MG, Roediger L, Koch JN, et al. Hydroxyethyl starch 130/0.4 and the risk of acute kidney injury after cardiopulmonary bypass: A single-center retrospective study. J of Cardiothorac and Vasc Anesth 2016: 30(4):869–75.
- 19.
- Wilkes MM, Navickis RJ. Postoperative renal replacement therapy after hydroxyethyl starch infusion: a meta-analysis of randomized trials. Neth J Crit Care 2014; 18:4–9.
- 20.
- Allen CJ, Valle EJ, Jouria JM, et al. Differences between blunt and penetrating trauma after resuscitation with HES. J Trauma Acute Care Surg 2014; 77(6):859–64.
- 21.
- Eriksson M, Brattström O, Mårtensson J, et al. Acute kidney injury following severe trauma: Risk factors and long-term outcome. J Trauma Acute Care Surg 2015; 79(3):407–12.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
6% Hetastarch in 0.9% Sodium Chloride Injection is supplied sterile and nonpyrogenic in 500 mL single-dose flexible plastic containers.
Unit of Sale Concentration NDC 0409-7248-03
30 g Hetastarch/500 mL
Case containing 12 Flexible Plastic Containers
(6 g Hetastarch/100 mL)
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Rx only
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1287-4.0
-
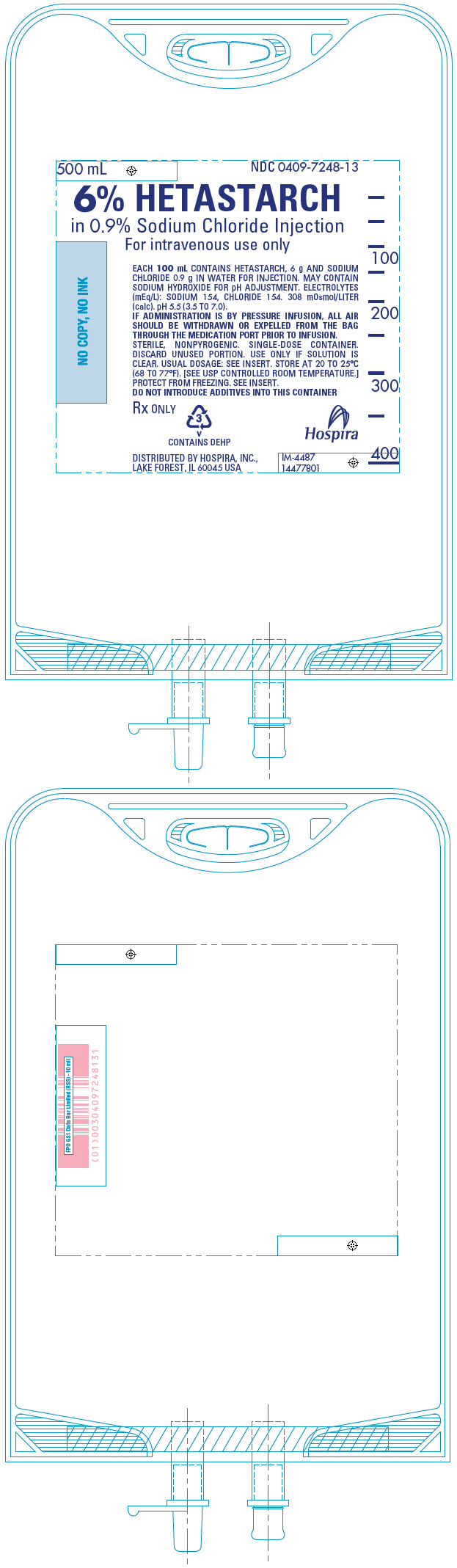
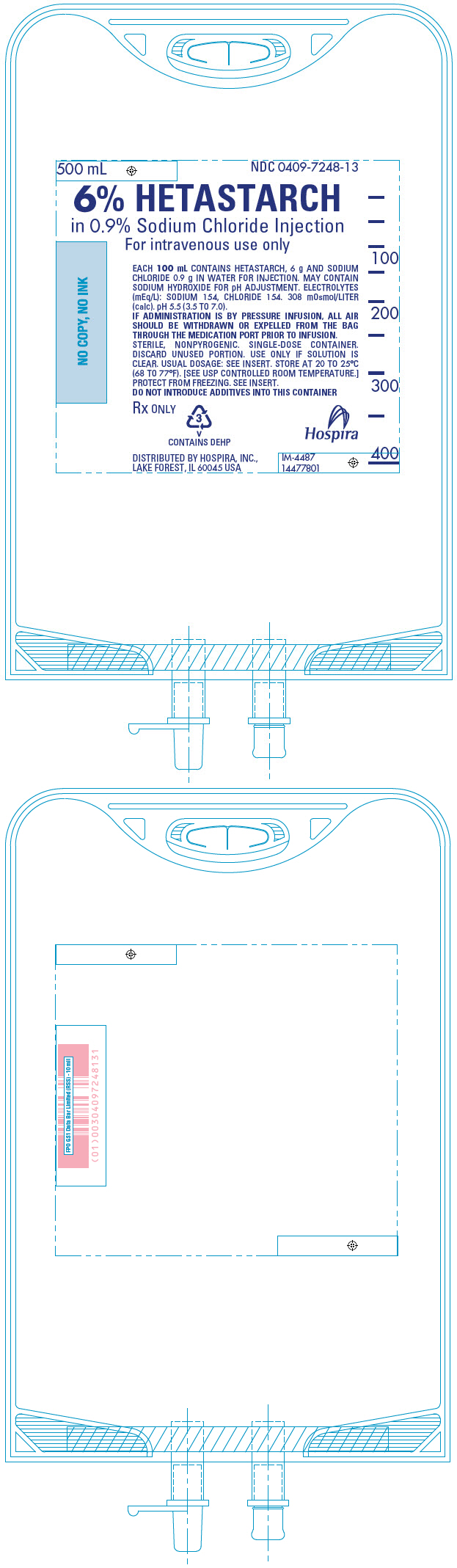
PRINCIPAL DISPLAY PANEL - 500 mL Bag
500 mL
NDC 0409-7248-136% HETASTARCH
in 0.9% Sodium Chloride Injection
For intravenous use onlyEACH 100 mL CONTAINS HETASTARCH, 6 g AND SODIUM
CHLORIDE 0.9 g IN WATER FOR INJECTION. MAY CONTAIN
SODIUM HYDROXIDE FOR pH ADJUSTMENT. ELECTROLYTES
(mEq/L): SODIUM 154, CHLORIDE 154. 308 mOsmol/LITER
(calc). pH 5.5 (3.5 TO 7.0).
IF ADMINISTRATION IS BY PRESSURE INFUSION, ALL AIR
SHOULD BE WITHDRAWN OR EXPELLED FROM THE BAG
THROUGH THE MEDICATION PORT PRIOR TO INFUSION.
STERILE, NONPYROGENIC. SINGLE-DOSE CONTAINER.
DISCARD UNUSED PORTION. USE ONLY IF SOLUTION IS
CLEAR. USUAL DOSAGE: SEE INSERT. STORE AT 20 TO 25°C
(68 TO 77°F). [SEE USP CONTROLLED ROOM TEMPERATURE.]
PROTECT FROM FREEZING. SEE INSERT.
DO NOT INTRODUCE ADDITIVES INTO THIS CONTAINERRx ONLY
3
V
CONTAINS DEHPDISTRIBUTED BY HOSPIRA, INC.,
LAKE FOREST, IL 60045 USAHospira
IM-4487
14477801 -
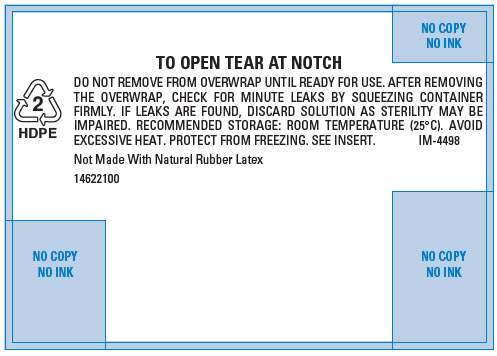
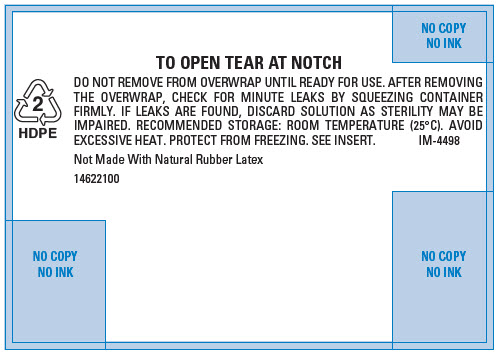
PRINCIPAL DISPLAY PANEL - 500 mL Bag Overwrap
2
HDPETO OPEN TEAR AT NOTCH
DO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING
THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER
FIRMLY. IF LEAKS ARE FOUND, DISCARD SOLUTION AS STERILITY MAY BE
IMPAIRED. RECOMMENDED STORAGE: ROOM TEMPERATURE (25°C). AVOID
EXCESSIVE HEAT. PROTECT FROM FREEZING. SEE INSERT.
IM-4498Not Made With Natural Rubber Latex
14622100
-
INGREDIENTS AND APPEARANCE
HETASTARCH IN SODIUM CHLORIDE
hetastarch injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-7248 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HETASTARCH (UNII: 875Y4127EA) (HETASTARCH - UNII:875Y4127EA) HETASTARCH 6 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.9 g in 100 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-7248-03 12 in 1 CASE 1 NDC:0409-7248-13 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA BA740193 02/28/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-7248) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-7248)