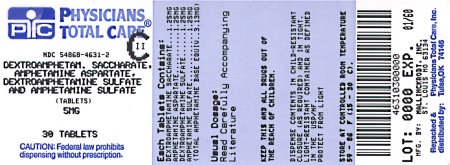
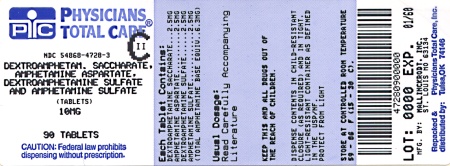
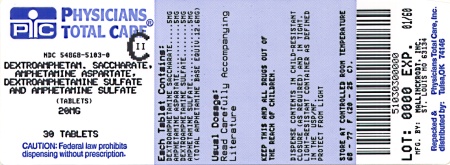
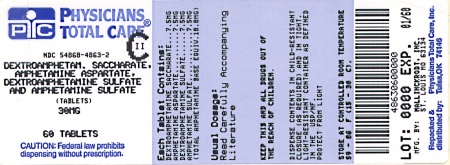
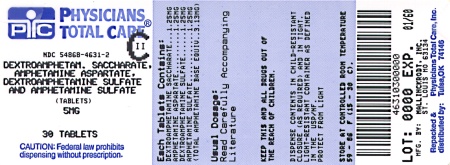
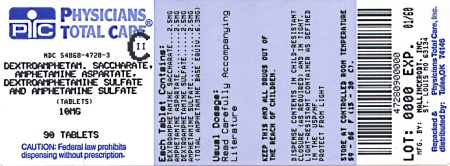
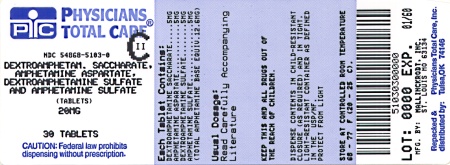
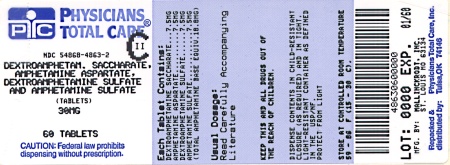
Label: DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-4631-0, 54868-4631-1, 54868-4631-2, 54868-4631-3, view more54868-4728-0, 54868-4728-1, 54868-4728-2, 54868-4728-3, 54868-4728-4, 54868-4728-5, 54868-4863-0, 54868-4863-1, 54868-4863-2, 54868-4863-3, 54868-5103-0, 54868-5103-1, 54868-5103-2, 54868-5103-3, 54868-5103-4, 54868-5103-5, 54868-5103-6, 54868-5387-0, 54868-5387-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
-
Source NDC Code(s):
0406-8886,
0406-8891,
0406-8892,
0406-8893,
view more0406-8894
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 9, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
AMPHETAMINES HAVE A HIGH POTENTIAL FOR ABUSE. ADMINISTRATION OF AMPHETAMINES FOR PROLONGED PERIODS OF TIME MAY LEAD TO DRUG DEPENDENCE AND MUST BE AVOIDED. PARTICULAR ATTENTION SHOULD BE PAID TO THE POSSIBILITY OF SUBJECTS OBTAINING AMPHETAMINES FOR NON-THERAPEUTIC USE OR DISTRIBUTION TO OTHERS, AND THE DRUGS SHOULD BE PRESCRIBED OR DISPENSED SPARINGLY.
MISUSE OF AMPHETAMINE MAY CAUSE SUDDEN DEATH AND SERIOUS CARDIOVASCULAR ADVERSE EVENTS.
-
DESCRIPTION
A single-entity amphetamine product combining the neutral sulfate salts of dextroamphetamine and amphetamine, with the dextro isomer of amphetamine saccharate and d, I-amphetamine aspartate. The structural formulas are as follows:
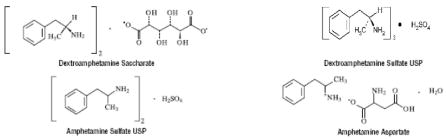
Each Tablet Contains:
5 mg
7.5 mg
10 mg
12.5 mg
15 mg
20 mg
30 mg
Dextroamphetamine Saccharate
1.25 mg
1.875 mg
2.5 mg
3.125 mg
3.75 mg
5 mg
7.5 mg
Amphetamine Aspartate
1.25 mg
1.875 mg
2.5 mg
3.125 mg
3.75 mg
5 mg
7.5 mg
Dextroamphetamine Sulfate USP
1.25 mg
1.875 mg
2.5 mg
3.125 mg
3.75 mg
5 mg
7.5 mg
Amphetamine Sulfate USP
1.25 mg
1.875 mg
2.5 mg
3.125 mg
3.75 mg
5 mg
7.5 mg
Total amphetamine
base equivalence
3.13 mg
4.75 mg
6.3 mg
7.8 mg
9.4 mg
12.6 mg
18.8 mg
In addition, each tablet contains the following inactive ingredients: Microcrystalline Cellulose NF, Silicon Dioxide NF, Povidone USP, and Stearic Acid NF.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known. Amphetamines are thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
Pharmacokinetics
Tablets of Mixed Salts of a Single Entity Amphetamine Product contain d-amphetamine and I-amphetamine salts in the ration of 3:1. Following administration of a single dose 10 or 30 mg of Tablets of Mixed Salts of a Single Entity Amphetamine Product to healthy volunteers under fasted conditions, peak plasma concentrations occurred approximately 3 hours post-dose for both d-amphetamine and I-amphetamine. The mean elimination half-life (t½) for d-amphetamine was shorter than the t½ of the l-isomer (9.77 to 11 hours vs. 11.5 to 13.8 hours). The PK parameters (Cmax, AUC0-inf) of d-and l-amphetamine increased approximately three-fold from 10 mg to 30 mg indicating dose-proportional pharmacokinetics.
The effect of food on the bioavailability of Tablets of Mixed Salts of a Single Entity Amphetamine Product has not been studied.
Metabolism and Excretion
Amphetamine is reported to be oxidized at the 4 position of the benzene ring to form 4-hydroxyamphetamine, or on the side chain α or β carbons to form alpha-hydroxy-amphetamine or norephedrine, respectively. Norephedrine and 4-hydroxy-amphetamine are both active and each is subsequently oxidized to form 4-hydroxy-norephedrine. Alpha-hydroxy-amphetamine undergoes deamination to form phenylacetone, which ultimately forms benzoic acid and its glucuronide and the glycine conjugate hippuric acid. Although the enzymes involved in amphetamine metabolism have not been clearly defined, CYP2D6 is known to be involved with formation of 4-hydroxy-amphetamine. Since CYP2D6 is genetically polymorphic, population variations in amphetamine metabolism are a possibility.
Amphetamine is known to inhibit monoamine oxidase, whereas the ability of amphetamine and its metabolites to inhibit various P450 isozymes and other enzymes has not been adequately elucidated. In vitro experiments with human microsomes indicate minor inhibition of CYP2D6 by amphetamine and minor inhibition of CYP1A2, 2D6, and 3A4 by one or more metabolites. However, due to the probability of auto-inhibition and the lack of information on the concentration of these metabolites relative to in vivo concentrations, no predications regarding the potential for amphetamine or its metabolites to inhibit the metabolism of other drugs by CYP isozymes in vivo can be made.
With normal urine pHs approximately half of an administered dose of amphetamine is recoverable in urine as derivatives of alpha-hydroxy-amphetamine and approximately another 30% to 40% of the dose is recoverable in urine as amphetamine itself. Since amphetamine has a pKa of 9.9, urinary recovery of amphetamine is highly dependent on pH and urine flow rates. Alkaline urine pHs result in less ionization and reduced renal elimination, and acidic pHs and high flow rates result in increased renal elimination with clearances greater than glomerular filtration rates, indicating the involvement of active secretion. Urinary recovery of amphetamine has been reported to range from 1% to 75%, depending on urinary pH, with the remaining fraction of the dose hepatically metabolized. Consequently, both hepatic and renal dysfunction have the potential to inhibit the elimination of amphetamine and result in prolonged exposures. In addition, drugs that effect urinary pH are known to alter the elimination of amphetamine, and any decrease in amphetamine's metabolism that might occur due to drug interactions or genetic polymorphisms is more likely to be clinically significant when renal elimination is decreased, (see PRECAUTIONS).
-
INDICATIONS AND USAGE
Tablets of Mixed Salts of a Single Entity Amphetamine product are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) and Narcolepsy.
Attention Deficit Hyperactivity Disorder (ADHD)
A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV®) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go;” excessive talking; blurting answers; can't wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.
Special Diagnostic Considerations- Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of the required number of DSM-IV® characterostocs/
Need for Comprehensive Treatment Program- Tablets of Mixed Salts of a Single Entity Amphetamine Product are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all children with this syndrome. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician's assessment of the chronicity and severity of the child's symptoms.
Long-Term Use- The effectiveness of Tablets of Mixed Salts of a Single Entity Amphetamine Product for long-term use has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use Tablets of Mixed Salts of a Single Entity Amphetamine Product for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
-
CONTRAINDICATIONS
Advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism, known hypersensitivity or idiosyncrasy to the sympathomimetic amines, glaucoma.
Agitated states.
Patients with a history of drug abuse.
During or within 14 days following the administration of monoamine oxidase inhibitors (hypertensive crises may result).
-
WARNINGS
Serious Cardiovascular Events
Sudden Death and Pre-existing Structural Cardiac Abnormalities or Other Serious Heart Problems
Children and Adolescents– Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some structural heart problems alone may carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug (see CONTRAINDICATIONS).
Adults– Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs (see CONTRAINDICATIONS).
Hypertension and Other Cardiovascular Conditions
Stimulant medications cause a modes increase in average blood pressure 9about 2 to 4 mmHg) and average heart rate (about 3 to 6 bpm) [see ADVERSE REACTIONS], and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia (see CONTRAINDICATIONS).Assessing Cardiovascular Status in Patients being Treated with Stimulant Medications.
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g. electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Pre-Existing Psychosis- Admiistration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with pre-existing psychotic disorder.
Bipolar Illness- Particular care should be taken i using stimulants to treat ADHD patients with comorbid comorbid bipolar disorder because of concern for possible induction of mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms- Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression- Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
Long-term Suppression of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they will likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining weight as expected may need to have their treatment interrupted.
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizure, in patients with prior EEG abnormalities in absence of seizures, and very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
-
PRECAUTIONS
General
The least amount of amphetamine feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage. Tablets of Mixed Salts of a Single Entity Amphetamine Product should be used with caution in patients who use other sympathomimetic drugs.
Tics
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in children and their families should precede use of stimulant medications.
Information for Patients
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machiner or vehicles; the patient should therefore be cautioned accordingly.
Drug Interactions
Acidifying agents - Gastrointestinal acidifying agents (guadethidine, reserpine, glutamic acid HCI, ascorbic acid fruit juices, etc.) lower absorption of amphetamines.
Urinary acidifying agents - (ammonium chloride, sodium acid phosphate, etc.) Increase the concentration of the ionized species of the amphetamine molecule, thereby increasing urinary excretion. Both groups of agents lower blood levels and efficacy of amphetamines.
Adrenergic blocker - Adrenergic blockers are inhibited by amphetamines.
Alkalinizing agents - Gastrointestinal alkalinizing agents (sodium bicarbonate, etc.) increase absorption of amphetamines. Co-administration of Tablets of Mixed Salts of a Single Entity Amphetamine Product and gastrointestinal alkalizing agents, such as antacids, should be avoided. Urinary alkalinizing agents (acetazolamide, some thiazides) increase the concentration of the non-ionized species of the amphetamine molecule, thereby decreasing urinary excretion. Both groups of agents increase blood levels and therefore potentiate the actions of amphetamines.
Antidepressants, tricyclic - Amphetamines may enhance the activity of tricyclic or sympathomimetic agents; d-amphetamine with desipramine or protriptyline and possibly other tricyclics cause striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated.
MAO inhibitors - MAOI antidepressants, as well as a metabolite of furazolidone, slow amphetamine metabolism. This slowing potentiates amphetamines, increasing their effect on the release of norepinephrine and other monoamines from adrenergic nerve endings; this can cause headaches and other signs of hypertensive crisis. A variety of neurological toxic effects and malignant hyperpyrexia can occur, sometimes with fatal results.
Antihistamines - Amphetamines may counteract the sedative effect of antihistamines.
Antihypertensives - Amphetamines may antagonize the hypotensive effects of antihypertensives.
Chlorpromazine - Chlorpromazine blocks dopamine and norepinephrine receptors, thus inhibiting the central stimulants effects of amphetamines, and can be used to treat amphetamine poisoning.
Ethosuximide - Amphetamines may delay intestinal absorption of ethosuximide.
Haloperidol - Haloperidol blocks dopamine receptors, thus inhibiting the central stimulant effects of amphetamines.
Lithium carbonate - The anorectic and stimulatory effects of amphetamines may be inhibited by lithium carbonate.
Meperidine - Amphetamines potentiate the analgesic effect of meperidine.
Methenamine therapy - Urinary excretion of amphetamines is increased, and efficacy is reduced, by acidifying agents used in methenamine therapy.
Norepinephrine - Amphetamines enhance the adrenergic effect of norepinephrine.
Phenytoin - Amphetaimes may delay intestinal absorption of phenytoin; co-administration of phenytoin may produce a synergistic anticonvulsant action.
Propoxyphene - In cases of propoxyphene overdosage, amphetamine CNS stimulation is potentiated and fatal convulsions can occur.
Veratrum alkaloids - Amphetamines inhibit the hypotensive effect of veratrum alkaloids.
Drug/Laboratory Test Interactions
Amphetamines can cause a significant elevation in plasma corticosteroid levels. This increase is greatest in the evening.Amphetamines may interfere with urinary steroid determinations.
Carcinogenesis/Mutagenesis and Impairment of Fertility
No evidence of carcinogenicity was found in studies in which d, I-amphetamine (enantiomer ration of 1:1) was administered to mice and rats in the diet for 2 years at doses of up to 30 mg/kg/day in male mice, 19 mg/kg/day in female mice, and 5 mg/kg/day in male and female rats. These doses are approximately 2.4, 1.5, and 0.8 times, respectively, the maximum recommended human dose of 30 mg/day [child] on a mg/m2 body surface area basis.
Amphetamine, in the enantiomer ratio present in Tablets of Mixed Salts of a Single Entity Amphetamine product (immediate-release)(d- to I- ratio of 3:1), was not clastogenic in the mouse bone marrow micronucleus test in vivo and was negative when tested in the E. coli component of the Ames test in vitro. d, I-Amphetamine (1:1 enantiomer ratio) has been reported to produce a positive response in the mouse bone marrow micronucleus test, an equivocal response in the Ames test, and negative responses in the in vitro sister chromatid exchange and chromosomal aberration assays.
Amphetamine, in the enantiomer ratio present in Tablets of Mixed Salts of a Single Entity Amphetamine product (immediate-release)(d- to I- ratio of 3:1), did not adversely affect fertility or early embryonic development in the rat at doses of up to 20 mg/kg/day (approximately 5 times the maximum recommended human dose of 30 mg/day on a mg/m2 body surface area basis).
Pregnancy
Teratogenic Effects. Pregnancy Category C - Amphetamine, in the enantiomer ratio present in Tablets of mixed Salts of a Single Entity Amphetamine product (d- to I- ratio of 3:1), had no apparent effects on embryofetal morphological development or survival when orally administered to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 6 and 16 mg/kg/day, respectively. These doses are approximately 1.5 and 8 times, respectively, the maximum recommended human dose of 30 mg/day [child] on a mg/m2 body surface area basis. Fetal malformations and death have been reported in mice following parenteral administration of d-amphetamine doses of 50 mg/kg/day (approximately 6 times that of a human dose of 30 mg/day [child] on a mg/m2 basis) or greater to pregnant animals. Administration of these doses was also associated with severe maternal toxicity.
A number of studies in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d,I-), at doses similar to those used clinically, can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
There are no adequate and well-controlled studies in pregnant women. There has been one report of severe congenital bony deformity, tracheo-esophageal fistula, and anal atresia (vater association) in a baby born to a woman who took dextroamphetamine sulfate with lovastatin during the first trimester of pregnancy. Amphetamines should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects - Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation, and significant lassitude.
Usage in Nursing Mothers - Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use - Long-term effects of amphetamines in children have not been well established. Amphetamines are not recommended for use in children under 3 years of age with Attention Deficit Hyperactivity Disorder described under INDICATIONS AND USAGE.
Geriatric Use - Tablets of Mixed Salts of a Single Entity Amphetamine Product have not been studied in the geriatric population.
-
ADVERSE REACTIONS
Cardiovascular– Palpitations, tachycardia, elevation of blood pressure, sudden death, myocardial infarction. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System– Psychotic episodes at recommended doses, overstimulation, restlessness, dizziness, insomnia, euphoria, dyskinesia, dysphoria, depression, tremor, headache, exacerbation of motor and phonic tics and Tourette's syndrome, seizures, stroke.
Gastrointestinal– Dryness of the mouth, unpleasant taste, diarrhea, constipation, other gastrointestinal disturbances. Anorexia and weight loss may occur as undesirable effects.
Allergic– Urticaria, rash, hypersensitivity reactions including angioedema and anaphylaxis. Serious skin rashes, including Stevens Johnson Syndrome and toxic epidermal necrolysis have been reported.
Endocrine– Impotence, changes in libido.
-
DRUG ABUSE AND DEPENDENCE
Dextroamphetamine sulfate is a Schedule II controlled substance.
Amphetamines have been extensively abused. Tolerance, extreme psychological dependence, and severe social disability have occurred. There are reports of patients who have increased the dosage to levels many times higher than recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with amphetamines include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes
-
OVERDOSAGE
Individual patient response to amphetamines varies widely. Toxic symptoms may occur idiosyncratically at low doses.
Symptoms
Manifestations of acute overdosage with amphetamines include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia and rhabdomyolysis.
Fatigue and depression usually follow the central stimulation.
Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse.
GASTROINTESTINAL SYMPTOMS INCLUDE NAUSEA, VOMITING DIARRHEA, AND ABDOMINAL CRAMPS. FATAL POISONING IS USUALLY PRECEDED BY CONVULSIONS AND COMA.
Treatment
CONSULT WITH A CERTIFIED POISON CONTROL CENTER FOR UP TO DATE GUIDANCE AND ADVISE. MANAGEMENT OF ACUTE AMPHETAMINE INTOXICATION IS LARGELY SYMPTOMATIC AND INCLUDES GASTRIC LAVAGE, ADMINISTRATION OF ACTIVATED CHARCOAL, ADMINISTRATION OF A CATHARTIC AND SEDATION. EXPERIENCE WITH HEMODIALYSIS OR PERITONEAL DIALYSIS IS INADEQUATE TO PERMIT RECOMMENDATION IN THIS REGARD. ACIDIFICATION OF THE URINE INCREASES AMPHETAMINE EXCRETION, BUT IS BELIEVED TO INCREASE RISK OF ACUTE RENAL FAILURE IF MYOGLOBINURIA IS PRESENT. IF ACUTE, SEVERE HYPERTENSION COMPLICATES AMPHETAMINE OVERDOSAGE, ADMINISTRATION OF INTRAVENOUS PHENTOLAMINE (REGITINE®, CIBA) HAS BEEN SUGGESTED. HOWEVER, A GRADUAL DROP IN BLOOD PRESSURE WILL USUALLY RESULT WHEN SUFFICIENT SEDATION HAS BEEN ACHIEVED. CHLORPROMAZINE ANTAGONIZES THE CENTRAL STIMULANT EFFECTS OF AMPHETAMINES AND CAN BE USED TO TREAT AMPHETAMINE INTOXICATION.
-
DOSAGE AND ADMINISTRATION
Regardless of indication, amphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted to the therapeutic needs and response of the patient. Late evening doses should be avoided because of the resulting insomnia.
Attention Deficit Hyperactivity Disorder
Not recommended for children under 3 years of age. In children from 3 to 5 years of age, start with 2.5 mg daily; daily dosage may be raised in increments of 2.5 mg at weekly intervals until optimal response is obtained.
In children 6 years of age and older, start with 5 mg once or twice daily; daily dosage may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. Only in rare cases will it be necessary to exceed a total of 40 mg per day. Give first dose on awakening; additional doses (1 or 2) at intervals of 4 to 6 hours.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
Narcolepsy
Usual dose 5 mg to 60 mg per day in divided doses, depending ion the individual patient response.
Narcolepsy seldom occurs in children under 12 years of age; however, when it does, dextroamphetamine sulfate may be used. The suggested initial dose for patients aged 6 to 12 is 5 mg daily; daily dose may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. In patients 12 years of age and older, start with 10 mg daily; daily dosage may be raised in increments of 10 mg at weekly intervals until optimal response is obtained. If bothersome adverse reactions appear (e.g., insomnia or anorexia), dosage should be reduced. Give first dose on awakening; additional doses (1 or 2) at intervals of 4 to 6 hours.
-
HOW SUPPLIED
Dextroamphetamine Saccharate, Amphetamine Aspartate, Dextroamphetamine Sulfate, Amphetamine Sulfate Tablets (Mixed Salts of a Single Entity Amphetamine Product) are supplied as follows:
5 mg:
White to cream colored pillow shaped tablet,
debossed with a “5” and a partial quadrisect on one side and a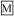 on the other side.
on the other side.
Bottles of 20 NDC 54868-4631-1
Bottles of 30 NDC 54868-4631-2
Bottles of 60 NDC 54868-4631-3
Bottles of 100 NDC 54868-4631-0
10 mg:
White to cream colored pillow shaped tablet,
debossed with a “10” and a partial quadrisect on one side and a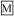 on the other side.
on the other side.
Bottles of 10 NDC 54868-4728-0
Bottles of 30 NDC 54868-4728-1
Bottles of 60 NDC 54868-4728-2
Bottles of 90 NDC 54868-4728-3
Bottles of 100 NDC 54868-4728-4
Bottles of 120 NDC 54868-4728-5
15 mg:
White to cream colored octagon shaped tablet,
debossed with a “15” and a partial quadrisect on one side and a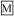
on the other side.
Bottles of 30 NDC 54868-5387-1
Bottles of 90 NDC 54868-5387-0
20 mg:
White to cream colored octagon shaped tablet,
debossed with a “20” and a partial quadrisect on one side and a on the other side.
on the other side.
Bottles of 10 NDC 54868-5103-4
Bottles of 20 NDC 54868-5103-3
Bottles of 30 NDC 54868-5103-0
Bottles of 60 NDC 54868-5103-1
Bottles of 90 NDC 54868-5103-2
Bottles of 100 NDC 54868-5103-6
Bottles of 120 NDC 54868-5103-5
30 mg:
White to cream colored octagon shaped tablet,
debossed with a “30” and a partial quadrisect on one side and a on the other side.
on the other side.
Bottles of 10 NDC 54868-4863-1
Bottles of 30 NDC 54868-4863-0
Bottles of 60 NDC 54868-4863-2
Bottles of 90 NDC 54868-4863-3
Dispense in a tight, light-resistant container with a child-resistant closure.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
 is a registered trademark of Mallinckrodt Inc.
is a registered trademark of Mallinckrodt Inc.tyco
Healthcare
MallinckrodtMallinckrodt Inc.
St. Louis, MO 63134 U.S.A.Printed in U.S.A.
Rev 092006
Repackaging and Relabeling by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PRINCIPAL DISPLAY PANEL
-
MEDICATION GUIDE
MEDICATION GUIDE
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE, DEXTROAMPHETAMINE SULFATE, and AMPHETAMINE SULFATE TABLETS CII
Read the Medication Guide that comes with dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about you or your child's treatment with dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets.
What is the most important information I should know about dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets?
The following have been reported with use of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate and other stimulant medicines.
1.Heart-related problems:
- sudden death in patients who have heart problems or heart defects
- stroke and heart attack in adults
- increased blood pressure and heart rate
Tell your doctor if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your doctor should check you or your child carefully for heart problems before starting dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate.
Your doctor should check you or your child's blood pressure and heart rate regularly during treatment with dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate.
Call your doctor right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate.
2.Mental (Psychiatric) problems:
All Patients
- new or worse behavior and thought problems
- new or worse bipolar illness
- new or worse aggressive behavior or hostility
Children and Teenagers
- new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your doctor right away if you or your child have any new or worsening mental symptoms or problems while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.
What is dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate?
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate is a central nervous system stimulant prescription medicine.
It is used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate is also used in the treatment of a sleep disorder called narcolepsy.
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets is a federally controlled substance (CII) because it can be abused or lead to dependence. Keep dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets in a safe place to prevent misuse and abuse. Selling or giving away dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets may harm others, and is against the law.
Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescriptions medicines or street drugs.
Who should not take dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets?
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets should not be taken if you or your child:
- have heart disease or hardening of the arteries
- have moderate to severe high blood pressure
- have hyperthyroidism
- have an eye problem called glaucoma
- are very anxious, tense, or agitated
- have a history of drug abuse
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor or MAOI.
- is sensitive to, allergic to, or had a reaction to other stimulant medicines
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets are not recommended for use in children less than 3 years old.
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate may not be right for you or your child. Before starting dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tell your or your child's doctor about all health conditions (or a family history of) including:
- heart problems, heart defects, high blood pressure
- mental problems including psychosis, mania, bipolar illness, or depression
- tics or Tourette's syndrome
- liver or kidney problems
- thyroid problems
- seizures or have had an abnormal brain wave test (EEG)
Tell your doctor if you or your child is pregnant, planning to become pregnant, or breastfeeding.
Can dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets be taken with other medicines?
Tell your doctor about all of the medicines that you or your child take including prescription and nonprescription medicines, vitamins, and herbal supplements.
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets.
Your doctor will decide whether dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets can be taken with other medicines.
Especially tell your doctor if you or your child takes:
- anti-depression medicines including MAOIs
- seizure medicines
- blood thinner medicines
- blood pressure medicines
- stomach acid medicines
- cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate without talking to your doctor first.
How should dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate be taken?
- Take dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
- Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets are usually taken two to three times a day. The first dose is usually taken when you first wake in the morning. One or two more doses may be taken during the day, 4 to 6 hours apart.
- Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets can be taken with or without food.
- From time to time, your doctor may stop dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate treatment for a while to check ADHD symptoms.
- Your doctor may do regular checks of the blood, heart, and blood pressure while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate. Children should have their height and weight checked often while taking dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate. Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate and amphetamine sulfate treatment may be stopped if a problem is found during these check-ups.
- If you or your child takes too much dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate?
See "What is the most important information I should know about dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate?" for information on reported heart and mental problems.
Other serious side effects include:
- slowing of growth (height and weight) in children
- seizures, mainly in patients with a history of seizures
- eyesight changes or blurred vision
Common side effects include:
- headache
- stomach ache
- trouble sleeping
- decreased appetite
- nervousness
- dizziness
Dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate may affect you or your child's ability to drive or do other dangerous activities.
Talk to your doctor if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information
How should I store dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets?
- Store dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate in a safe place at room temperature, 20° to 25°C (68° to 77°F).
- Keep dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate and all medicines out of the reach of children.
General information about dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate for a condition for which it was not prescribed. Do not give dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate that was written for healthcare professionals. For more information, you may also contact Mallinckrodt Inc.., (the maker of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets) at 1-800-325-8888.
What are the ingredients in dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablets?
Active Ingredients: dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate
Inactive Ingredients: Microcrystalline Cellulose NF, Silicon Dioxide NF, Povidone USP, and Stearic Acid NF.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
INGREDIENTS AND APPEARANCE
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4631(NDC:0406-8891) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 1.25 mg AMPHETAMINE ASPARTATE (UNII: H527KAP6L5) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE 1.25 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 1.25 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 1.25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to cream colored) Score 4 pieces Shape SQUARE Size 6mm Flavor Imprint Code 5;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4631-0 100 in 1 BOTTLE, PLASTIC 2 NDC:54868-4631-1 20 in 1 BOTTLE, PLASTIC 3 NDC:54868-4631-2 30 in 1 BOTTLE, PLASTIC 4 NDC:54868-4631-3 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040440 06/04/2002 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4728(NDC:0406-8892) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 2.5 mg AMPHETAMINE ASPARTATE (UNII: H527KAP6L5) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE 2.5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 2.5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 2.5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to cream colored) Score 4 pieces Shape SQUARE Size 6mm Flavor Imprint Code 10;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4728-0 10 in 1 BOTTLE, PLASTIC 2 NDC:54868-4728-1 30 in 1 BOTTLE, PLASTIC 3 NDC:54868-4728-2 60 in 1 BOTTLE, PLASTIC 4 NDC:54868-4728-3 90 in 1 BOTTLE, PLASTIC 5 NDC:54868-4728-4 100 in 1 BOTTLE, PLASTIC 6 NDC:54868-4728-5 120 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040440 01/17/2003 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5387(NDC:0406-8886) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 3.75 mg AMPHETAMINE ASPARTATE (UNII: H527KAP6L5) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE 3.75 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 3.75 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 3.75 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to cream colored) Score 4 pieces Shape OCTAGON (8 sided) Size 6mm Flavor Imprint Code 12;5;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5387-0 90 in 1 BOTTLE 2 NDC:54868-5387-1 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040440 08/11/2005 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5103(NDC:0406-8893) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 5 mg AMPHETAMINE ASPARTATE (UNII: H527KAP6L5) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE 5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to cream colored) Score 4 pieces Shape OCTAGON (8 sided) Size 8mm Flavor Imprint Code 20;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5103-0 30 in 1 BOTTLE 2 NDC:54868-5103-1 60 in 1 BOTTLE 3 NDC:54868-5103-2 90 in 1 BOTTLE 4 NDC:54868-5103-3 20 in 1 BOTTLE 5 NDC:54868-5103-4 10 in 1 BOTTLE 6 NDC:54868-5103-5 120 in 1 BOTTLE 7 NDC:54868-5103-6 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040440 07/07/2004 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4863(NDC:0406-8894) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 7.5 mg AMPHETAMINE ASPARTATE (UNII: H527KAP6L5) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE 7.5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 7.5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 7.5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to cream colored) Score 4 pieces Shape OCTAGON (8 sided) Size 9mm Flavor Imprint Code 30;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4863-0 30 in 1 BOTTLE 2 NDC:54868-4863-1 10 in 1 BOTTLE 3 NDC:54868-4863-2 60 in 1 BOTTLE 4 NDC:54868-4863-3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040440 07/25/2003 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel(54868-4631, 54868-4728, 54868-5387, 54868-5103, 54868-4863) , repack(54868-4631, 54868-4728, 54868-5387, 54868-5103, 54868-4863)
View Labeling Archives for this drug
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE tablet
Number of versions: 5
RxNorm
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE tablet
Get Label RSS Feed for this Drug
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE tablet
NDC Codes
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.