Label: DEXILANT- dexlansoprazole capsule, delayed release
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-6152-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 64764-175
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 8, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXILANT safely and effectively. See full prescribing information for DEXILANT.
DEXILANT (dexlansoprazole) delayed-release capsules, for oral use
Initial U.S. Approval: 1995 (lansoprazole)RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Healing of EE: 60 mg once daily for up to 8 weeks. (2.1)
- Maintenance of healed EE: 30 mg once daily for up to 6 months. (2.1)
- Symptomatic non-erosive GERD: 30 mg once daily for 4 weeks. (2.1)
- Hepatic impairment: Consider 30 mg maximum daily dose for patients with moderate hepatic impairment (Child-Pugh Class B). No studies were conducted in patients with severe hepatic impairment (Child-Pugh Class C). (2.2, 8.7)
- DEXILANT can be taken without regard to food. (2.3)
- DEXILANT should be swallowed whole. Alternatively, capsules can be opened, sprinkled on one tablespoon of applesauce, and swallowed immediately. (2.3)
DOSAGE FORMS AND STRENGTHS
- Delayed-Release Capsules: 30 mg and 60 mg. (3)
CONTRAINDICATIONS
Patients with known hypersensitivity to any component of the formulation. (4)
WARNINGS AND PRECAUTIONS
- Gastric malignancy: Symptomatic response with DEXILANT does not preclude the presence of gastric malignancy. (5.1)
- Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.2)
- Hypomagnesemia: Hypomagnesemia has been reported rarely with prolonged treatment with PPIs. (5.3)
ADVERSE REACTIONS
Most commonly reported adverse reactions (≥2%): diarrhea, abdominal pain, nausea, upper respiratory tract infection, vomiting, and flatulence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Atazanavir: Do not co-administer with DEXILANT because atazanavir systemic concentrations may be substantially decreased. (7.1)
- Drugs with pH-dependent absorption (e.g., ampicillin esters, digoxin, iron salts, ketoconazole): DEXILANT may interfere with absorption of drugs for which gastric pH is important for bioavailability. (7.1)
- Warfarin: Patients taking concomitant warfarin may require monitoring for increases in international normalized ratio (INR) and prothrombin time. (7.2)
- Tacrolimus: Concomitant tacrolimus use may increase tacrolimus whole blood concentrations. (7.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2012
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Healing of Erosive Esophagitis
1.2 Maintenance of Healed Erosive Esophagitis
1.3 Symptomatic Non-Erosive Gastroesophageal Reflux Disease
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Hepatic Impairment
2.3 Important Administration Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastric Malignancy
5.2 Bone Fracture
5.3 Hypomagnesemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs with pH-Dependent Absorption Pharmacokinetics
7.2 Warfarin
7.3 Tacrolimus
7.4 Clopidogrel
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Healing of Erosive Esophagitis
14.2 Maintenance of Healed Erosive Esophagitis
14.3 Symptomatic Non-Erosive GERD
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
DEXILANT is available as capsules in 30 mg and 60 mg strengths for adult use. Directions for use in each indication are summarized in Table 1.
Table 1: DEXILANT Dosing Recommendations Indication Recommended Dose Frequency Healing of EE 60 mg Once daily for up to 8 weeks Maintenance of Healed EE and relief of heartburn 30 mg Once daily Symptomatic Non-Erosive GERD 30 mg Once daily for 4 weeks 2.2 Hepatic Impairment
No adjustment for DEXILANT is necessary for patients with mild hepatic impairment (Child-Pugh Class A). Consider a maximum daily dose of 30 mg for patients with moderate hepatic impairment (Child-Pugh Class B). No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.3 Important Administration Information
DEXILANT can be taken without regard to food.
DEXILANT should be swallowed whole.
- Alternatively, DEXILANT capsules can be administered as follows:
- –Open capsule;
- –Sprinkle intact granules on one tablespoon of applesauce;
- – Swallow immediately. Granules should not be chewed.
- Alternatively, DEXILANT capsules can be administered as follows:
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DEXILANT is contraindicated in patients with known hypersensitivity to any component of the formulation [see Description (11)]. Hypersensitivity and anaphylaxis have been reported with DEXILANT use [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastric Malignancy
Symptomatic response with DEXILANT does not preclude the presence of gastric malignancy.
5.2 Bone Fracture
Several published observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration (2) and Adverse Reactions (6)].
5.3 Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of DEXILANT was evaluated in 4548 patients in controlled and uncontrolled clinical studies, including 863 patients treated for at least 6 months and 203 patients treated for one year. Patients ranged in age from 18 to 90 years (median age 48 years), with 54% female, 85% Caucasian, 8% Black, 4% Asian, and 3% other races. Six randomized controlled clinical trials were conducted for the treatment of EE, maintenance of healed EE, and symptomatic GERD, which included 896 patients on placebo, 455 patients on DEXILANT 30 mg, 2218 patients on DEXILANT 60 mg, and 1363 patients on lansoprazole 30 mg once daily.
Most Commonly Reported Adverse Reactions
The most common adverse reactions (≥2%) that occurred at a higher incidence for DEXILANT than placebo in the controlled studies are presented in Table 2.
Table 2: Incidence of Adverse Reactions in Controlled Studies Placebo DEXILANT
30 mgDEXILANT
60 mgDEXILANT
TotalLansoprazole
30 mgAdverse Reaction (N=896)
%(N=455)
%(N=2218)
%(N=2621)
%(N=1363)
%Diarrhea 2.9 5.1 4.7 4.8 3.2 Abdominal Pain 3.5 3.5 4.0 4.0 2.6 Nausea 2.6 3.3 2.8 2.9 1.8 Upper Respiratory Tract Infection 0.8 2.9 1.7 1.9 0.8 Vomiting 0.8 2.2 1.4 1.6 1.1 Flatulence 0.6 2.6 1.4 1.6 1.2 Adverse Reactions Resulting in Discontinuation
In controlled clinical studies, the most common adverse reaction leading to discontinuation from DEXILANT therapy was diarrhea (0.7%).
Other Adverse Reactions
Other adverse reactions that were reported in controlled studies at an incidence of less than 2% are listed below by body system:
Blood and Lymphatic System Disorders: anemia, lymphadenopathy
Cardiac Disorders: angina, arrhythmia, bradycardia, chest pain, edema, myocardial infarction, palpitation, tachycardia
Ear and Labyrinth Disorders: ear pain, tinnitus, vertigo
Endocrine Disorders: goiter
Eye Disorders: eye irritation, eye swelling
Gastrointestinal Disorders: abdominal discomfort, abdominal tenderness, abnormal feces, anal discomfort, Barrett's esophagus, bezoar, bowel sounds abnormal, breath odor, colitis microscopic, colonic polyp, constipation, dry mouth, duodenitis, dyspepsia, dysphagia, enteritis, eructation, esophagitis, gastric polyp, gastritis, gastroenteritis, gastrointestinal disorders, gastrointestinal hypermotility disorders, GERD, GI ulcers and perforation, hematemesis, hematochezia, hemorrhoids, impaired gastric emptying, irritable bowel syndrome, mucus stools, oral mucosal blistering, painful defecation, proctitis, paresthesia oral, rectal hemorrhage, retching
General Disorders and Administration Site Conditions: adverse drug reaction, asthenia, chest pain, chills, feeling abnormal, inflammation, mucosal inflammation, nodule, pain, pyrexia
Hepatobiliary Disorders: biliary colic, cholelithiasis, hepatomegaly
Immune System Disorders: hypersensitivity
Infections and Infestations: candida infections, influenza, nasopharyngitis, oral herpes, pharyngitis, sinusitis, viral infection, vulvo-vaginal infection
Injury, Poisoning and Procedural Complications: falls, fractures, joint sprains, overdose, procedural pain, sunburn
Laboratory Investigations: ALP increased, ALT increased, AST increased, bilirubin decreased/increased, blood creatinine increased, blood gastrin increased, blood glucose increased, blood potassium increased, liver function test abnormal, platelet count decreased, total protein increased, weight increase
Metabolism and Nutrition Disorders: appetite changes, hypercalcemia, hypokalemia
Musculoskeletal and Connective Tissue Disorders: arthralgia, arthritis, muscle cramps, musculoskeletal pain, myalgia
Nervous System Disorders: altered taste, convulsion, dizziness, headaches, migraine, memory impairment, paresthesia, psychomotor hyperactivity, tremor, trigeminal neuralgia
Psychiatric Disorders: abnormal dreams, anxiety, depression, insomnia, libido changes
Renal and Urinary Disorders: dysuria, micturition urgency
Reproductive System and Breast Disorders: dysmenorrhea, dyspareunia, menorrhagia, menstrual disorder
Respiratory, Thoracic and Mediastinal Disorders: aspiration, asthma, bronchitis, cough, dyspnoea, hiccups, hyperventilation, respiratory tract congestion, sore throat
Skin and Subcutaneous Tissue Disorders: acne, dermatitis, erythema, pruritis, rash, skin lesion, urticaria
Vascular Disorders: deep vein thrombosis, hot flush, hypertension
Additional adverse reactions that were reported in a long-term uncontrolled study and were considered related to DEXILANT by the treating physician included: anaphylaxis, auditory hallucination, B-cell lymphoma, bursitis, central obesity, cholecystitis acute, dehydration, diabetes mellitus, dysphonia, epistaxis, folliculitis, gout, herpes zoster, hyperlipidemia, hypothyroidism, increased neutrophils, MCHC decrease, neutropenia, rectal tenesmus, restless legs syndrome, somnolence, tonsillitis.
Other adverse reactions not observed with DEXILANT, but occurring with the racemate lansoprazole can be found in the lansoprazole prescribing information, ADVERSE REACTIONS section.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval of DEXILANT. As these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura
Ear and Labyrinth Disorders: deafness
Eye Disorders: blurred vision
Gastrointestinal Disorders: oral edema, pancreatitis
General Disorders and Administration Site Conditions: facial edema
Hepatobiliary Disorders: drug-induced hepatitis
Immune System Disorders: anaphylactic shock (requiring emergency intervention), exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal)
Metabolism and Nutrition Disorders: hypomagnesemia, hyponatremia
Musculoskeletal System Disorders: bone fracture
Nervous System Disorders: cerebrovascular accident, transient ischemic attack
Renal and Urinary Disorders: acute renal failure
Respiratory, Thoracic and Mediastinal Disorders: pharyngeal edema, throat tightness
Skin and Subcutaneous Tissue Disorders: generalized rash, leucocytoclastic vasculitis
-
7 DRUG INTERACTIONS
7.1 Drugs with pH-Dependent Absorption Pharmacokinetics
DEXILANT causes inhibition of gastric acid secretion. DEXILANT is likely to substantially decrease the systemic concentrations of the HIV protease inhibitor atazanavir, which is dependent upon the presence of gastric acid for absorption, and may result in a loss of therapeutic effect of atazanavir and the development of HIV resistance. Therefore, DEXILANT should not be co-administered with atazanavir.
DEXILANT may interfere with the absorption of other drugs where gastric pH is an important determinant of oral bioavailability (e.g., ampicillin esters, digoxin, iron salts, ketoconazole).
7.2 Warfarin
Co-administration of DEXILANT 90 mg and warfarin 25 mg did not affect the pharmacokinetics of warfarin or INR [see Clinical Pharmacology (12.3)]. However, there have been reports of increased INR and prothrombin time in patients receiving PPIs and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with DEXILANT and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
7.3 Tacrolimus
Concomitant administration of dexlansoprazole and tacrolimus may increase whole blood levels of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
7.4 Clopidogrel
Concomitant administration of dexlansoprazole and clopidogrel in healthy subjects had no clinically important effect on exposure to the active metabolite of clopidogrel or clopidogrel-induced platelet inhibition [see Clinical Pharmacology (12.3)]. No dose adjustment of clopidogrel is necessary when administered with an approved dose of DEXILANT.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category B. There are no adequate and well-controlled studies with dexlansoprazole in pregnant women. There were no adverse fetal effects in animal reproduction studies of dexlansoprazole in rabbits. Because animal reproduction studies are not always predictive of human response, DEXILANT should be used during pregnancy only if clearly needed.
A reproduction study conducted in rabbits at oral dexlansoprazole doses up to approximately 9 times the maximum recommended human dexlansoprazole dose (60 mg per day) revealed no evidence of impaired fertility or harm to the fetus due to dexlansoprazole. In addition, reproduction studies performed in pregnant rats with oral lansoprazole at doses up to 40 times the recommended human lansoprazole dose and in pregnant rabbits at oral lansoprazole doses up to 16 times the recommended human lansoprazole dose revealed no evidence of impaired fertility or harm to the fetus due to lansoprazole [see Nonclinical Toxicology (13.2)].
8.3 Nursing Mothers
It is not known whether dexlansoprazole is excreted in human milk. However, lansoprazole and its metabolites are present in rat milk following the administration of lansoprazole. As many drugs are excreted in human milk, and because of the potential for tumorigenicity shown for lansoprazole in rat carcinogenicity studies [see Nonclinical Toxicology (13.1)], a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of DEXILANT in pediatric patients (less than 18 years of age) have not been established.
8.5 Geriatric Use
In clinical studies of DEXILANT, 11% of patients were aged 65 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified significant differences in responses between geriatric and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of DEXILANT is necessary in patients with renal impairment. The pharmacokinetics of dexlansoprazole in patients with renal impairment are not expected to be altered since dexlansoprazole is extensively metabolized in the liver to inactive metabolites, and no parent drug is recovered in the urine following an oral dose of dexlansoprazole [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment for DEXILANT is necessary for patients with mild hepatic impairment (Child-Pugh Class A). DEXILANT 30 mg should be considered for patients with moderate hepatic impairment (Child-Pugh Class B). No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C) [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There have been no reports of significant overdose of DEXILANT. Multiple doses of DEXILANT 120 mg and a single dose of DEXILANT 300 mg did not result in death or other severe adverse events. However, serious adverse events of hypertension have been reported in association with twice daily doses of DEXILANT 60 mg. Non-serious adverse reactions observed with twice daily doses of DEXILANT 60 mg include hot flashes, contusion, oropharyngeal pain, and weight loss. Dexlansoprazole is not expected to be removed from the circulation by hemodialysis. If an overdose occurs, treatment should be symptomatic and supportive.
-
11 DESCRIPTION
The active ingredient in DEXILANT (dexlansoprazole) delayed-release capsules, a proton pump inhibitor, is (+)-2-[(R)-{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl] methyl} sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Dexlansoprazole is the R-enantiomer of lansoprazole (a racemic mixture of the R- and S-enantiomers). Its empirical formula is: C16H14F3N3O2S, with a molecular weight of 369.36. The structural formula is:
Dexlansoprazole is a white to nearly white crystalline powder which melts with decomposition at 140°C. Dexlansoprazole is freely soluble in dimethylformamide, methanol, dichloromethane, ethanol, and ethyl acetate; and soluble in acetonitrile; slightly soluble in ether; and very slightly soluble in water; and practically insoluble in hexane.
Dexlansoprazole is stable when exposed to light. Dexlansoprazole is more stable in neutral and alkaline conditions than acidic conditions.
DEXILANT is supplied as a dual delayed-release formulation in capsules for oral administration. The capsules contain dexlansoprazole in a mixture of two types of enteric-coated granules with different pH-dependent dissolution profiles [see Clinical Pharmacology (12.3)].
DEXILANT is available in two dosage strengths: 30 mg and 60 mg, per capsule. Each capsule contains enteric-coated granules consisting of dexlansoprazole (active ingredient) and the following inactive ingredients: sugar spheres, magnesium carbonate, sucrose, low-substituted hydroxypropyl cellulose, titanium dioxide, hydroxypropyl cellulose, hypromellose 2910, talc, methacrylic acid copolymers, polyethylene glycol 8000, triethyl citrate, polysorbate 80, and colloidal silicon dioxide. The components of the capsule shell include the following inactive ingredients: hypromellose, carrageenan and potassium chloride. Based on the capsule shell color, blue contains FD&C Blue No. 2 aluminum lake; gray contains black ferric oxide; and both contain titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dexlansoprazole is a PPI that suppresses gastric acid secretion by specific inhibition of the (H+,K+)-ATPase in the gastric parietal cell. By acting specifically on the proton pump, dexlansoprazole blocks the final step of acid production.
12.2 Pharmacodynamics
Antisecretory Activity
The effects of DEXILANT 60 mg (n=20) or lansoprazole 30 mg (n=23) once daily for five days on 24-hour intragastric pH were assessed in healthy subjects in a multiple-dose crossover study. The results are summarized in Table 3.
Table 3: Effect on 24-hour Intragastric pH on Day 5 After Administration of DEXILANT or Lansoprazole DEXILANT 60 mg Lansoprazole 30 mg Mean Intragastric pH 4.55 4.13 % Time Intragastric pH > 4
(hours)71
(17 hours)60
(14 hours)Serum Gastrin Effects
The effect of DEXILANT on serum gastrin concentrations was evaluated in approximately 3460 patients in clinical trials up to 8 weeks and in 1023 patients for up to 6 to 12 months. The mean fasting gastrin concentrations increased from baseline during treatment with DEXILANT 30 mg and 60 mg doses. In patients treated for more than 6 months, mean serum gastrin levels increased during approximately the first 3 months of treatment and were stable for the remainder of treatment. Mean serum gastrin levels returned to pre-treatment levels within one month of discontinuation of treatment.
Enterochromaffin-Like Cell (ECL) Effects
There were no reports of ECL cell hyperplasia in gastric biopsy specimens obtained from 653 patients treated with DEXILANT 30 mg, 60 mg or 90 mg for up to 12 months.
During lifetime exposure of rats dosed daily with up to 150 mg per kg per day of lansoprazole, marked hypergastrinemia was observed followed by ECL cell proliferation and formation of carcinoid tumors, especially in female rats [see Nonclinical Toxicology (13.1)].
Effect on Cardiac Repolarization
A study was conducted to assess the potential of DEXILANT to prolong the QT/QTc interval in healthy adult subjects. DEXILANT doses of 90 mg or 300 mg did not delay cardiac repolarization compared to placebo. The positive control (moxifloxacin) produced statistically significantly greater mean maximum and time-averaged QT/QTc intervals compared to placebo.
12.3 Pharmacokinetics
The dual delayed release formulation of DEXILANT results in a dexlansoprazole plasma concentration-time profile with two distinct peaks; the first peak occurs 1 to 2 hours after administration, followed by a second peak within 4 to 5 hours (see Figure 1). Dexlansoprazole is eliminated with a half-life of approximately 1 to 2 hours in healthy subjects and in patients with symptomatic GERD. No accumulation of dexlansoprazole occurs after multiple, once daily doses of DEXILANT 30 mg or 60 mg, although mean AUCt and Cmax values of dexlansoprazole were slightly higher (less than 10%) on day 5 than on day 1.
Figure 1: Mean Plasma Dexlansoprazole Concentration –
Time Profile Following Oral Administration of 30 or 60 mg DEXILANT
Once Daily for 5 Days in Healthy Subjects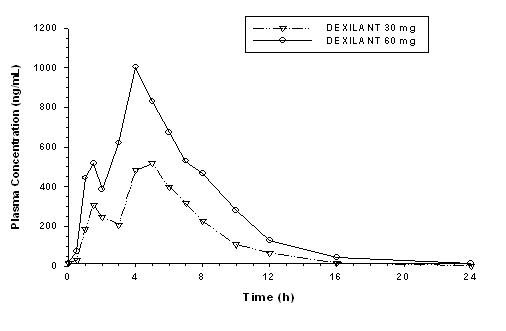
The pharmacokinetics of dexlansoprazole are highly variable, with percent coefficient of variation (CV%) values for Cmax, AUC, and CL/F of greater than 30% (see Table 4).
Table 4: Mean (CV%) Pharmacokinetic Parameters for Subjects on Day 5 After Administration of DEXILANT Dose
(mg)Cmax
(ng/mL)AUC24
(ng∙h/mL)CL/F
(L/h)30 658 (40%)
(N=44)3275 (47%)
(N=43)11.4 (48%)
(N=43)60 1397 (51%)
(N=79)6529 (60%)
(N=73)11.6 (46%)
(N=41)Absorption
After oral administration of DEXILANT 30 mg or 60 mg to healthy subjects and symptomatic GERD patients, mean Cmax and AUC values of dexlansoprazole increased approximately dose proportionally (see Figure 1).
Distribution
Plasma protein binding of dexlansoprazole ranged from 96.1% to 98.8% in healthy subjects and was independent of concentration from 0.01 to 20 mcg per mL. The apparent volume of distribution (Vz/F) after multiple doses in symptomatic GERD patients was 40.3 L.
Metabolism
Dexlansoprazole is extensively metabolized in the liver by oxidation, reduction, and subsequent formation of sulfate, glucuronide and glutathione conjugates to inactive metabolites. Oxidative metabolites are formed by the cytochrome P450 (CYP) enzyme system including hydroxylation mainly by CYP2C19, and oxidation to the sulfone by CYP3A4.
CYP2C19 is a polymorphic liver enzyme which exhibits three phenotypes in the metabolism of CYP2C19 substrates; extensive metabolizers (*1/*1), intermediate metabolizers (*1/mutant) and poor metabolizers (mutant/mutant). Dexlansoprazole is the major circulating component in plasma regardless of CYP2C19 metabolizer status. In CYP2C19 intermediate and extensive metabolizers, the major plasma metabolites are 5-hydroxy dexlansoprazole and its glucuronide conjugate, while in CYP2C19 poor metabolizers dexlansoprazole sulfone is the major plasma metabolite.
Elimination
Following the administration of DEXILANT, no unchanged dexlansoprazole is excreted in urine. Following the administration of [14C]dexlansoprazole to 6 healthy male subjects, approximately 50.7% (standard deviation (SD): 9.0%) of the administered radioactivity was excreted in urine and 47.6% (SD: 7.3%) in the feces. Apparent clearance (CL/F) in healthy subjects was 11.4 to 11.6 L/h, respectively, after 5-days of 30 or 60 mg once daily administration.
Effect of CYP2C19 Polymorphism on Systemic Exposure of Dexlansoprazole
Systemic exposure of dexlansoprazole is generally higher in intermediate and poor metabolizers. In male Japanese subjects who received a single dose of DEXILANT 30 mg or 60 mg (N=2 to 6 subjects/group), mean dexlansoprazole Cmax and AUC values were up to 2 times higher in intermediate compared to extensive metabolizers; in poor metabolizers, mean Cmax was up to 4 times higher and mean AUC was up to 12 times higher compared to extensive metabolizers. Though such study was not conducted in Caucasians and African Americans, it is expected dexlansoprazole exposure in these races will be affected by CYP2C19 phenotypes as well.
Effect of Food on Pharmacokinetics and Pharmacodynamics
In food-effect studies in healthy subjects receiving DEXILANT under various fed conditions compared to fasting, increases in Cmax ranged from 12% to 55%, increases in AUC ranged from 9% to 37%, and tmax varied (ranging from a decrease of 0.7 hours to an increase of 3 hours). No significant differences in mean intragastric pH were observed between fasted and various fed conditions. However, the percentage of time intragastric pH exceeded 4 over the 24-hour dosing interval decreased slightly when DEXILANT was administered after a meal (57%) relative to fasting (64%), primarily due to a decreased response in intragastric pH during the first 4 hours after dosing. Because of this, while DEXILANT can be taken without regard to food, some patients may benefit from administering the dose prior to a meal if post-meal symptoms do not resolve under post-fed conditions.
Special Populations
Pediatric Use
The pharmacokinetics of dexlansoprazole in patients under the age of 18 years have not been studied.
Geriatric Use
The terminal elimination half-life of dexlansoprazole is significantly increased in geriatric subjects compared to younger subjects (2.23 and 1.5 hours, respectively); this difference is not clinically relevant. Dexlansoprazole exhibited higher systemic exposure (AUC) in geriatric subjects (34.5% higher) than younger subjects. No dosage adjustment is needed in geriatric patients [see Use in Specific Populations (8.5)].
Renal Impairment
Dexlansoprazole is extensively metabolized in the liver to inactive metabolites, and no parent drug is recovered in the urine following an oral dose of dexlansoprazole. Therefore, the pharmacokinetics of dexlansoprazole are not expected to be altered in patients with renal impairment, and no studies were conducted in subjects with renal impairment [see Use in Specific Populations (8.6)]. In addition, the pharmacokinetics of lansoprazole were studied in patients with mild, moderate or severe renal impairment; results demonstrated no need for a dose adjustment for this patient population.
Hepatic Impairment
In a study of 12 patients with moderately impaired hepatic function who received a single oral dose of DEXILANT 60 mg, plasma exposure (AUC) of bound and unbound dexlansoprazole in the hepatic impairment group was approximately 2 times greater compared to subjects with normal hepatic function. This difference in exposure was not due to a difference in protein binding between the two liver function groups. No adjustment for DEXILANT is necessary for patients with mild hepatic impairment (Child-Pugh Class A). DEXILANT 30 mg should be considered for patients with moderate hepatic impairment (Child-Pugh Class B). No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7)].
Drug-Drug Interactions
Warfarin
In a study of 20 healthy subjects, co-administration of DEXILANT 90 mg once daily for 11 days with a single 25 mg oral dose of warfarin on day 6 did not result in any significant differences in the pharmacokinetics of warfarin or INR compared to administration of warfarin with placebo. However, there have been reports of increased INR and prothrombin time in patients receiving PPIs and warfarin concomitantly [see Drug Interactions (7.2)].
Cytochrome P 450 Interactions
Dexlansoprazole is metabolized, in part, by CYP2C19 and CYP3A4 [see Clinical Pharmacology (12.3)].
In vitro studies have shown that dexlansoprazole is not likely to inhibit CYP isoforms 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 2E1 or 3A4. As such, no clinically relevant interactions with drugs metabolized by these CYP enzymes would be expected. Furthermore, in vivo studies showed that DEXILANT did not have an impact on the pharmacokinetics of coadministered phenytoin (CYP2C9 substrate) or theophylline (CYP1A2 substrate). The subjects' CYP1A2 genotypes in the drug-drug interaction study with theophylline were not determined. Although in vitro studies indicated that DEXILANT has the potential to inhibit CYP2C19 in vivo, an in vivo drug-drug interaction study in mainly CYP2C19 extensive and intermediate metabolizers has shown that DEXILANT does not affect the pharmacokinetics of diazepam (CYP2C19 substrate).
Clopidogrel
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. A study of healthy subjects who were CYP2C19 extensive metabolizers, receiving once daily administration of clopidogrel 75 mg alone or concomitantly with DEXILANT 60 mg (n=40), for 9 days was conducted. The mean AUC of the active metabolite of clopidogrel was reduced by approximately 9% (mean AUC ratio was 91%, with 90% CI of 86-97%) when DEXILANT was coadministered compared to administration of clopidogrel alone. Pharmacodynamic parameters were also measured and demonstrated that the change in inhibition of platelet aggregation (induced by 5 mcM ADP) was related to the change in the exposure to clopidogrel active metabolite. The clinical significance of this finding is not clear.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of dexlansoprazole was assessed using lansoprazole studies. In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated orally with lansoprazole at doses of 5 to 150 mg per kg per day, about 1 to 40 times the exposure on a body surface (mg/m2) basis of a 50 kg person of average height [1.46 m2 body surface area (BSA)] given the recommended human dose of lansoprazole 30 mg per day.
Lansoprazole produced dose-related gastric ECL cell hyperplasia and ECL cell carcinoids in both male and female rats [see Clinical Pharmacology (12.2)].
In rats, lansoprazole also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg per kg per day (4 to 40 times the recommended human lansoprazole dose based on BSA) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat.
In a 24-month carcinogenicity study, CD-1 mice were treated orally with lansoprazole doses of 15 to 600 mg per kg per day, 2 to 80 times the recommended human lansoprazole dose based on BSA. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg lansoprazole per kg per day (40 to 80 times the recommended human lansoprazole dose based on BSA) and female mice treated with 150 to 600 mg lansoprazole per kg per day (20 to 80 times the recommended human lansoprazole dose based on BSA) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg per kg per day (10 to 80 times the recommended human lansoprazole dose based on BSA).
A 26-week p53 (+/-) transgenic mouse carcinogenicity study of lansoprazole was not positive.
Lansoprazole was positive in the Ames test and the in vitro human lymphocyte chromosomal aberration assay. Lansoprazole was not genotoxic in the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test or the rat bone marrow cell chromosomal aberration test.
Dexlansoprazole was positive in the Ames test and in the in vitro chromosome aberration test using Chinese hamster lung cells. Dexlansoprazole was negative in the in vivo mouse micronucleus test.
The potential effects of dexlansoprazole on fertility and reproductive performance were assessed using lansoprazole studies. Lansoprazole at oral doses up to 150 mg per kg per day (40 times the recommended human lansoprazole dose based on BSA) was found to have no effect on fertility and reproductive performance of male and female rats.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
A reproduction study conducted in rabbits at oral dexlansoprazole doses up to 30 mg per kg per day (approximately 9 times the maximum recommended human dexlansoprazole dose [60 mg per day] based on BSA) revealed no evidence of impaired fertility or harm to the fetus due to dexlansoprazole. In addition, reproduction studies performed in pregnant rats with oral lansoprazole at doses up to 150 mg per kg per day (40 times the recommended human lansoprazole dose based on BSA) and in pregnant rabbits at oral lansoprazole doses up to 30 mg per kg per day (16 times the recommended human lansoprazole dose based on BSA) revealed no evidence of impaired fertility or harm to the fetus due to lansoprazole.
-
14 CLINICAL STUDIES
14.1 Healing of Erosive Esophagitis
Two multi-center, double-blind, active-controlled, randomized, 8-week studies were conducted in patients with endoscopically confirmed EE. Severity of the disease was classified based on the Los Angeles Classification Grading System (Grades A-D). Patients were randomized to one of the following three treatment groups: DEXILANT 60 mg daily, DEXILANT 90 mg daily or lansoprazole 30 mg daily. Patients who were H. pylori positive or who had Barrett's Esophagus and/or definite dysplastic changes at baseline were excluded from these studies. A total of 4092 patients were enrolled and ranged in age from 18 to 90 years (median age 48 years) with 54% male. Race was distributed as follows: 87% Caucasian, 5% Black and 8% other. Based on the Los Angeles Classification, 71% of patients had mild EE (Grades A and B) and 29% of patients had moderate to severe EE (Grades C and D) before treatment.
The studies were designed to test non-inferiority. If non-inferiority was demonstrated then superiority would be tested. Although non-inferiority was demonstrated in both studies, the finding of superiority in one study was not replicated in the other.
The proportion of patients with healed EE at week 4 or 8 is presented below in Table 5.
Table 5: EE Healing Rates: All Grades Study Number of Patients
(N)Treatment Group
(daily)Week 4
% HealedWeek 8
% Healed(95% CI) for the Treatment Difference (DEXILANT– Lansoprazole) by Week 8 CI = Confidence interval - *
- Demonstrated non-inferiority to lansoprazole
1 657 DEXILANT 60 mg 70 87 (-1.5, 6.1)* 648 Lansoprazole 30 mg 65 85 2 639 DEXILANT 60 mg 66 85 (2.2, 10.5)* 656 Lansoprazole 30 mg 65 79 DEXILANT 90 mg was studied and did not provide additional clinical benefit over DEXILANT 60 mg.
14.2 Maintenance of Healed Erosive Esophagitis
A multi-center, double-blind, placebo-controlled, randomized study was conducted in patients who successfully completed an EE study and showed endoscopically confirmed healed EE. Maintenance of healing and symptom resolution over a six-month period were evaluated with DEXILANT 30 mg or 60 mg once daily compared to placebo. A total of 445 patients were enrolled and ranged in age from 18 to 85 years (median age 49 years), with 52% female. Race was distributed as follows: 90% Caucasian, 5% Black and 5% other.
Sixty-six percent of patients treated with 30 mg of DEXILANT remained healed over the six-month time period as confirmed by endoscopy (see Table 6).
Table 6: Maintenance Rates of Healed EE at Month 6 Number of Patients
(N)Treatment Group
(daily)Maintenance Rate
(%)125 DEXILANT 30 mg 66.4 119 Placebo 14.3 DEXILANT 60 mg was studied and did not provide additional clinical benefit over DEXILANT 30 mg.
The effect of DEXILANT 30 mg on maintenance of relief of heartburn was also evaluated. Upon entry into the maintenance study, a majority of patients' baseline heartburn severity was rated as none. DEXILANT 30 mg demonstrated a statistically significantly higher percent of 24-hour heartburn-free periods compared to placebo over the 6-month treatment period (see Table 7). The majority of patients treated with placebo discontinued due to relapse of EE between month two and month six.
Table 7: Median Percentage of 24-Hour Heartburn-Free Periods of the Maintenance of Healed EE Study Overall Treatment Month 1 Month 6 Treatment Group
(daily)N Heartburn-Free 24-hour Periods
(%)N Heartburn-Free 24-hour Periods
(%)N Heartburn-Free 24-hour Periods
(%)DEXILANT 30 mg 132 96.1 126 96.7 80 98.3 Placebo 141 28.6 117 28.6 23 73.3 14.3 Symptomatic Non-Erosive GERD
A multi-center, double-blind, placebo-controlled, randomized, 4-week study was conducted in patients with a diagnosis of symptomatic non-erosive GERD made primarily by presentation of symptoms. These patients who identified heartburn as their primary symptom, had a history of heartburn for 6 months or longer, had heartburn on at least 4 of 7 days immediately prior to randomization and had no esophageal erosions as confirmed by endoscopy. However, patients with symptoms which were not acid-related may not have been excluded using these inclusion criteria. Patients were randomized to one of the following treatment groups: DEXILANT 30 mg daily, 60 mg daily, or placebo. A total of 947 patients were enrolled and ranged in age from 18 to 86 years (median age 48 years) with 71% female. Race was distributed as follows: 82% Caucasian, 14% Black and 4% other.
DEXILANT 30 mg provided statistically significantly greater percent of days with heartburn-free 24-hour periods over placebo as assessed by daily diary over 4 weeks (see Table 8). DEXILANT 60 mg was studied and provided no additional clinical benefit over DEXILANT 30 mg.
Table 8: Median Percentages of 24-Hour Heartburn-Free Periods During the 4 Week Treatment Period of the Symptomatic Non-Erosive GERD Study N Treatment Group
(daily)Heartburn-Free
24-hour Periods
(%)312 DEXILANT 30 mg 54.9 310 Placebo 18.5 A higher percentage of patients on DEXILANT 30 mg had heartburn-free 24-hour periods compared to placebo as early as the first three days of treatment and this was sustained throughout the treatment period (percentage of patients on Day 3: DEXILANT 38% versus placebo 15%; on Day 28: DEXILANT 63% versus placebo 40%).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
"See FDA-Approved Patient Labeling (Patient Information)"
To ensure the safe and effective use of DEXILANT, this information and instructions provided in the FDA-approved Patient Information Leaflet should be discussed with the patient.
Inform the patient to watch for signs of an allergic reaction as these could be serious and may require that DEXILANT be discontinued.
Advise the patient to immediately report and seek care for any cardiovascular or neurological symptoms including palpitations, dizziness, seizures, and tetany as these may be signs of hypomagnesemia [see Warnings and Precautions (5.3)].
Advise the patient to tell their health care provider if they take atazanavir, tacrolimus, warfarin and drugs that are affected by gastric pH changes [see Drug Interactions (7)]
Advise the patient to follow the dosing instructions in the Patient Information Leaflet and inform the patient that:
- DEXILANT is available as a delayed-release capsule.
- DEXILANT may be taken without regard to food.
- DEXILANT should be swallowed whole.
- Alternatively, DEXILANT capsules can be administered as follows:
- –Open capsule;
- –Sprinkle intact granules on one tablespoon of applesauce;
- –Swallow immediately. Granules should not be chewed.
- –Do not store for later use.
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Patient Information
DEXILANT (decks-ĭ-launt)
(dexlansoprazole)
delayed-release capsulesRead the Patient Information that comes with DEXILANT before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is DEXILANT?
DEXILANT is a prescription medicine called a proton pump inhibitor (PPI). DEXILANT reduces the amount of acid in your stomach.
DEXILANT is used in adults:
- for up to 8 weeks to heal acid-related damage to the lining of the esophagus (called erosive esophagitis or EE).
- for up to 6 months to continue healing of erosive esophagitis and relief of heartburn.
- for 4 weeks to treat heartburn related to gastroesophageal reflux disease (GERD).
GERD happens when acid from your stomach enters the tube (esophagus) that connects your mouth to your stomach. This may cause a burning feeling in your chest or throat, sour taste or burping.
In some cases, acid can damage the lining of your esophagus. This damage is called erosive esophagitis or EE.
DEXILANT may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your doctor.
It is not known if DEXILANT is safe and effective in children under 18 years of age.
Who should not take DEXILANT?
Do not take DEXILANT if you are allergic to DEXILANT or any of its ingredients. See the end of this leaflet for a complete list of ingredients in DEXILANT.
What should I tell my doctor before taking DEXILANT?
Before you take DEXILANT, tell your doctor if you:
- have been told that you have low magnesium levels in your blood
- have liver problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if DEXILANT will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breast-feeding or planning to breast-feed. You and your doctor should decide if you will take DEXILANT or breast-feed. You should not do both without first talking with your doctor.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. DEXILANT may affect how other medicines work, and other medicines may affect how DEXILANT works. Especially tell your doctor if you take:
- ampicillin sodium (Unasyn) or ampicillin trihydrate (Principen)
- atazanavir (Reyataz)
- digoxin (Lanoxin)
- a product that contains iron
- ketoconazole (Nizoral)
- warfarin (Coumadin, Jantoven)
- tacrolimus (Prograf)
Ask your doctor or pharmacist if you are not sure if your medicine is listed above.
How should I take DEXILANT?
- Take DEXILANT exactly as prescribed by your doctor.
- Do not change your dose or stop taking DEXILANT without talking to your doctor first.
- You can take DEXILANT with or without food.
- Swallow DEXILANT capsules whole.
- If you have trouble swallowing DEXILANT capsules whole, you can open the capsules and sprinkle the contents on a tablespoon of applesauce. Be sure to swallow the applesauce mixture right away. Do not chew the mixture. Do not store for later use.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time. If you are not sure about dosing, call your doctor.
- If you take too much DEXILANT, call your doctor right away or go to the nearest hospital or emergency room.
What are the possible side effects of DEXILANT?
The most common side effects of DEXILANT include:
- diarrhea
- stomach pain
- nausea
- common cold
- vomiting
- gas
Other side effects
Serious allergic reactions. Tell your doctor if you get any of the following symptoms with DEXILANT:
- rash
- face swelling
- throat tightness
- difficulty breathing
Your doctor may stop DEXILANT if these symptoms happen.
- Low magnesium levels in your body. This problem can be serious. Low magnesium can happen in some people who take a proton pump inhibitor medicine for at least 3 months. If low magnesium levels happen, it is usually after a year of treatment. You may or may not have symptoms of low magnesium.
Tell your doctor right away if you have any of these symptoms:
- seizures
- dizziness
- abnormal or fast heartbeat
- jitteriness
- jerking movements or shaking (tremors)
- muscle weakness
- spasms of the hands and feet
- cramps or muscle aches
- spasm of the voice box
Your doctor may check the level of magnesium in your body before you start taking DEXILANT, or during treatment, if you will be taking DEXILANT for a long period of time.
Bone fractures. People who are taking multiple daily doses of proton pump inhibitor medicines for a long period of time may have an increased risk of fractures of the hip, wrist or spine.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of DEXILANT. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store DEXILANT?
- Store DEXILANT at room temperature between 59°F to 86°F (15°C to 30°C).
Keep DEXILANT and all medicines out of the reach of children.
General information about DEXILANT
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use DEXILANT for a condition for which it was not prescribed. Do not give DEXILANT to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information Leaflet provides a summary of the most important information about DEXILANT. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
For more information, go to www.DEXILANT.com or call 1-877-825-3327.
What are the ingredients in DEXILANT?
Active ingredient: dexlansoprazole.
Inactive ingredients: sugar spheres, magnesium carbonate, sucrose, low-substituted hydroxypropyl cellulose, titanium dioxide, hydroxypropyl cellulose, hypromellose 2910, talc, methacrylic acid copolymers, polyethylene glycol 8000, triethyl citrate, polysorbate 80, and colloidal silicon dioxide. The capsule shell is made of hypromellose, carrageenan and potassium chloride. Based on the capsule shell color, blue contains FD&C Blue No. 2 aluminum lake; gray contains black ferric oxide; and both contain titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
-
SPL UNCLASSIFIED SECTION
Distributed by
Takeda Pharmaceuticals America, Inc.
Deerfield, IL 60015DEXILANT is a trademark of Takeda Pharmaceuticals North America, Inc. and used under license by Takeda Pharmaceuticals America, Inc. Trademark registered with the U.S. Patent and Trademark office.
All other trademark names are the property of their respective owners.
©2009-2011 Takeda Pharmaceuticals America, Inc.
DEX006 R15
Revised: October 2011
Additional barcode labeling by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEXILANT
dexlansoprazole capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-6152(NDC:64764-175) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength dexlansoprazole (UNII: UYE4T5I70X) (dexlansoprazole - UNII:UYE4T5I70X) dexlansoprazole 60 mg Inactive Ingredients Ingredient Name Strength Methacrylic Acid - Methyl Methacrylate Copolymer (1:1) (UNII: 74G4R6TH13) Methacrylic Acid - Methyl Methacrylate Copolymer (1:2) (UNII: 5KY68S2577) Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type A (UNII: NX76LV5T8J) magnesium carbonate (UNII: 0E53J927NA) sucrose (UNII: C151H8M554) hydroxypropyl cellulose, low substituted (UNII: 2165RE0K14) titanium dioxide (UNII: 15FIX9V2JP) hydroxypropyl cellulose (UNII: RFW2ET671P) hypromelloses (UNII: 3NXW29V3WO) talc (UNII: 7SEV7J4R1U) polyethylene glycol 8000 (UNII: Q662QK8M3B) triethyl citrate (UNII: 8Z96QXD6UM) polysorbate 80 (UNII: 6OZP39ZG8H) silicon dioxide (UNII: ETJ7Z6XBU4) carrageenan (UNII: 5C69YCD2YJ) potassium chloride (UNII: 660YQ98I10) aluminum oxide (UNII: LMI26O6933) FD&C Blue No. 2 (UNII: L06K8R7DQK) Product Characteristics Color BLUE (opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code TAP;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-6152-0 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022287 08/19/2010 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel


