Label: SAPHNELO- anifrolumab-fnia injection, solution
- NDC Code(s): 0310-3040-00
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SAPHNELO® safely and effectively. See full prescribing information for SAPHNELO.
SAPHNELO (anifrolumab-fnia) injection, for intravenous use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
INDICATIONS AND USAGE
SAPHNELO is a type I interferon (IFN) receptor antagonist indicated for the treatment of adult patients with moderate to severe systemic lupus erythematosus (SLE), who are receiving standard therapy. (1)
Limitations of Use: The efficacy of SAPHNELO has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. Use of SAPHNELO is not recommended in these situations. (1)
DOSAGE AND ADMINISTRATION
The recommended dosage is 300 mg as an intravenous infusion over a 30‑minute period every 4 weeks. For complete dilution and intravenous administration instructions see Full Prescribing Information. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 300 mg/2 mL (150 mg/mL) in a single-dose vial. (3)
CONTRAINDICATIONS
SAPHNELO is contraindicated in patients with a history of anaphylaxis with anifrolumab-fnia. (4)
WARNINGS AND PRECAUTIONS
- •
- Serious Infections: Serious and sometimes fatal infections have occurred in patients receiving SAPHNELO. SAPHNELO increases the risk of respiratory infections and herpes zoster. Avoid initiating treatment during an active infection. Consider the individual benefit-risk if using in patients with severe or chronic infections. Consider interrupting therapy with SAPHNELO if patients develop a new infection during treatment. (5.1)
- •
- Hypersensitivity Reactions Including Anaphylaxis: Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported. (5.2)
- •
- Malignancy: Consider the individual benefit-risk in patients with known risk factors for malignancy prior to prescribing SAPHNELO. (5.3)
- •
- Immunizations: Avoid use of live or live-attenuated vaccines in patients receiving SAPHNELO. (5.4)
- •
- Not Recommended for Use with Other Biologic Therapies. (5.5)
ADVERSE REACTIONS
Most common adverse drug reactions (incidence ≥5%) are nasopharyngitis, upper respiratory tract infections, bronchitis, infusion related reactions, herpes zoster and cough. (6)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Recommendations
2.2 Instructions for Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Hypersensitivity Reactions Including Anaphylaxis
5.3 Malignancy
5.4 Immunizations
5.5 Not Recommended for Concomitant Use with Other Biologic Therapies
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
SAPHNELO (anifrolumab-fnia) is indicated for the treatment of adult patients with moderate to severe systemic lupus erythematosus (SLE), who are receiving standard therapy [see Clinical Studies (14)].
Limitations of Use
The efficacy of SAPHNELO has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. Use of SAPHNELO is not recommended in these situations.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Recommendations
SAPHNELO must be diluted prior to intravenous administration [see Dosage and Administration (2.2)].
The recommended dosage of SAPHNELO is 300 mg, administered as an intravenous infusion over a 30-minute period, every 4 weeks.
Missed dose
If a planned infusion is missed, administer SAPHNELO as soon as possible. Maintain a minimum interval of 14 days between infusions.
2.2 Instructions for Preparation and Administration
SAPHNELO is supplied as a single-dose vial. Prepare the diluted infusion solution using aseptic technique, by the following procedure:
- 1.
- Visually inspect the vial for particulate matter and discoloration. SAPHNELO is a clear to opalescent, colorless to slightly yellow, solution. Discard the vial if the solution is cloudy, discolored or visible particles are observed. Do not shake the vial.
- 2.
- Withdraw and discard 2 mL of solution from a 50 mL or 100 mL 0.9% Sodium Chloride Injection, USP infusion bag.
- 3.
- Withdraw 2 mL of solution from the vial of SAPHNELO and add it to the infusion bag. Mix the solution by gentle inversion. Do not shake.
- 4.
- Each vial is intended for one time use only. Discard any unused portion remaining in the vial.
- 5.
- Administer the infusion solution immediately after preparation.
- 6.
- If the infusion solution is not administered immediately, store the diluted solution of SAPHNELO at room temperature (59°F to 77°F, 15°C to 25°C) for up to 4 hours, or refrigerated (36°F to 46°F, 2°C to 8°C) for up to 24 hours. Do not freeze. Protect from light. If refrigerated, allow the diluted SAPHNELO solution to reach room temperature prior to administration.
- 7.
- Administer the infusion solution intravenously over a 30-minute period through an infusion line containing a sterile, low-protein binding 0.2 to 15 micron in-line or add-on filter.
- 8.
- To ensure the complete dose of SAPHNELO has been administered, flush the entire infusion line with 25 mL of 0.9% Sodium Chloride Injection, USP at the end of the infusion.
- 9.
- Do not co-administer other medicinal products through the same infusion line.
- 10.
- Dispose of any unused medicinal product or waste material in accordance with local requirements.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SAPHNELO is contraindicated in patients with a history of anaphylaxis with anifrolumab-fnia [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Serious and sometimes fatal infections (including COVID‑19) have occurred in patients receiving immunosuppressive agents, including SAPHNELO. Overall, the incidence of serious infections in controlled trials was similar in patients receiving SAPHNELO compared with placebo, whereas fatal infections occurred more frequently in patients receiving SAPHNELO [see Adverse Reactions (6.1)].
In controlled trials, SAPHNELO increased the risk of respiratory infections and herpes zoster (disseminated herpes zoster events have been reported) [see Adverse Reactions (6.1)].
Consider the benefit and risk of administering SAPHNELO in patients with a chronic infection, a history of recurrent infections, or known risk factors for infection. Avoid initiating treatment with SAPHNELO in patients with any clinically significant active infection until the infection resolves or is adequately treated. Instruct patients to seek medical advice if signs or symptoms of clinically significant infection occur. If a patient develops an infection, or is not responding to standard anti-infective therapy, monitor the patient closely and consider interrupting SAPHNELO therapy until the infection resolves.
5.2 Hypersensitivity Reactions Including Anaphylaxis
Serious hypersensitivity reactions (including anaphylaxis) have been reported following SAPHNELO administration [see Contraindication (4)]. Events of angioedema have also been reported [see Adverse Reactions (6.1)].
Other hypersensitivity reactions and infusion-related reactions have occurred following administration of SAPHNELO [see Adverse Reactions (6.1)]. Consider pre-medication before infusion of SAPHNELO for patients with a history of these reactions.
SAPHNELO should be administered by healthcare providers prepared to manage hypersensitivity reactions, including anaphylaxis, and infusion-related reactions. If a serious infusion-related or hypersensitivity reaction (e.g., anaphylaxis) occurs, immediately interrupt the administration of SAPHNELO and initiate appropriate therapy.
5.3 Malignancy
There is an increased risk of malignancies with the use of immunosuppressants. The impact of SAPHNELO treatment on the potential development of malignancies is not known.
Consider the individual benefit-risk in patients with known risk factors for the development or reoccurrence of malignancy prior to prescribing SAPHNELO. In patients who develop malignancies, consider the benefit-risk of continued treatment with SAPHNELO.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are also discussed elsewhere in the labeling:
- •
- Serious Infections [see Warnings and Precautions (5.1)]
- •
- Hypersensitivity Reactions Including Anaphylaxis [see Warnings and Precautions (5.2)]
- •
- Malignancy [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SAPHNELO was assessed through 52 weeks in patients with moderate to severe SLE who received anifrolumab-fnia 300 mg by intravenous infusion every 4 weeks (N=459), compared to placebo (N=466) in controlled clinical trials (Trials 1, 2 and 3) [see Clinical Studies (14)]. The population studied had a mean age of 41 years (range: 18 to 69), of which 93% were female, 60% White, 13% Black/African American, and 10% Asian.
In the controlled-clinical trials, adverse reactions, irrespective of causality, were reported in 87% of patients receiving SAPHNELO and 79% of patients receiving placebo.
Adverse reactions that occurred at greater than or equal to 2% incidence are shown in Table 1.
Table 1 Adverse Reactions Occurring in ≥2% of Patients on SAPHNELO 300 mg (Trials 1, 2 and 3) at 52 weeks - *
- Upper respiratory tract infections (including Upper respiratory tract infections, Nasopharyngitis, Pharyngitis)
- †
- Bronchitis (including Bronchitis, Bronchitis viral, Tracheobronchitis)
- ‡
- Respiratory tract infection (including Respiratory tract infection, Respiratory tract infection viral, Respiratory tract infection bacterial)
Adverse Reaction
SAPHNELO
(N=459)
%
Placebo
(N=466)
%
Upper respiratory tract infection*
34
23
Bronchitis†
11
5.2
Infusion‑related reactions
9.4
7.1
Herpes Zoster
6.1
1.3
Cough
5.0
3.2
Respiratory tract infection‡
3.3
1.5
Hypersensitivity
2.8
0.6
All patients received standard therapy.
Long-term Safety
Patients who completed Trials 2 and 3 (Phase III feeder trials) were eligible to continue on treatment in a randomized, double-blind, placebo-controlled long-term extension (LTE) study, for an additional 3 years. The long-term safety of SAPHNELO was assessed in 257 patients who received anifrolumab-fnia 300 mg and 112 patients who received placebo in both a feeder trial and the LTE. Of these, 177 patients who received SAPHNELO (68.9%) and 52 patients who received placebo (46.4%) completed a total of 4 years on treatment. The overall long-term safety profile of SAPHNELO was consistent with Trials 1, 2 and 3.
Specific Adverse Reactions
Infections
In the 52‑week controlled-clinical trials, infections were reported in a greater proportion of patients while on treatment with SAPHNELO compared to placebo (69.7% [320/459] versus 55.4% [258/466]), corresponding to exposure-adjusted incidence rates (EAIR) of 141.8 and 99.9 per 100 patient years (PY), respectively.
Serious Infections
In the 52‑week controlled-clinical trials, the incidence of serious infections while on treatment was 4.8% (22/459) in patients treated with SAPHNELO compared with 5.6% (26/466) in patients receiving placebo, corresponding to EAIR of 5.4 and 6.6 per 100 PY, respectively. The most frequent serious infection was pneumonia.
In the 52‑week controlled-clinical trials, fatal infections occurred in 0.4% of patients receiving SAPHNELO and 0.2% of the patients receiving placebo.
During the LTE study, the most common serious infections were COVID-19 and pneumonia.
Herpes Zoster
In the 52‑week controlled-clinical trials, the incidence of herpes zoster in patients while on treatment with SAPHNELO was 6.1% (28/459) and 1.3% (6/466) in patients on placebo, corresponding to EAIRs of 6.9 and 1.5 per 100 PY, respectively. Cases with multidermatomal involvement and disseminated presentation have been reported. Of the 28 SAPHNELO-treated patients with herpes zoster, 2 experienced disseminated disease requiring hospitalization compared to none among patients who received placebo.
Hypersensitivity Reactions Including Anaphylaxis
During the SLE development program, there was one report of an anaphylactic reaction in a patient who received 150 mg anifrolumab-fnia, and 4 reports of angioedema after 300 mg. In general, the hypersensitivity reactions were predominantly mild or moderate in intensity and did not lead to discontinuation of SAPHNELO.
In the 52‑week controlled-clinical trials, hypersensitivity reactions occurred in 2.8% (13/459) of patients while on treatment with SAPHNELO and 0.6% (3/466) of patients on placebo, corresponding to EAIR of 3.2 and 0.7 per 100 PY, respectively. Serious hypersensitivity reactions were reported for 0.6% (3/459) of patients receiving SAPHNELO, including angioedema (n=2).
Infusion-related Reactions
Infusion-related reactions were mild to moderate in intensity; the most common symptoms were headache, nausea, vomiting, fatigue, and dizziness.
In the 52‑week controlled-clinical trials, the incidence of infusion-related reactions while on treatment was 9.4% (43/459) in patients while on treatment with SAPHNELO and 7.1% (33/466) in patients on placebo, corresponding to EAIRs of 11.1 and 8.7 per 100 PY, respectively.
Malignancies
In 52‑week controlled-clinical trials, malignancies (excluding non-melanoma skin cancers) were observed in 0.7% (3/459) and 0.6% (3/466) of patients receiving SAPHNELO and placebo, corresponding to EAIR of 0.7 and 0.7 per 100 PY, respectively. Malignant neoplasm (including non-melanoma skin cancers) was reported for 1.3% (6/459) patients receiving SAPHNELO, compared to 0.6% (3/466) patients receiving placebo (EAIR: 1.3 and 0.7 per 100 PY, respectively). The malignancies that were reported in more than one patient treated with SAPHNELO included breast cancer and squamous cell carcinoma.
6.2 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of SAPHNELO. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Arthralgia
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to SAPHNELO during pregnancy. For more information about the registry or to report a pregnancy while on SAPHNELO, contact AstraZeneca at 1‑877‑693‑9268.
Risk Summary
The limited human data with SAPHNELO use in pregnant women are insufficient to inform on drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcome. Monoclonal IgG antibodies are known to be actively transported across the placenta as pregnancy progresses; therefore, anifrolumab-fnia exposure to the fetus may be greater during the third trimester of pregnancy.
In an enhanced pre- and post-natal development study with pregnant cynomolgus monkeys that received intravenous administration of anifrolumab-fnia, there was no evidence of embryotoxicity or fetal malformations with exposures up to approximately 28‑times the exposure at the maximum recommended human dose (MRHD) on an Area Under Curve (AUC) basis (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Pregnant women with SLE are at increased risk of adverse pregnancy outcomes, including worsening of the underlying disease, premature birth, miscarriage, and intrauterine growth restriction. Maternal lupus nephritis increases the risk of hypertension and preeclampsia/eclampsia. Passage of maternal autoantibodies across the placenta may result in adverse neonatal outcomes, including neonatal lupus and congenital heart block.
Animal Data: In an enhanced pre- and post-natal development study, pregnant cynomolgus monkeys received anifrolumab-fnia at intravenous doses of 30 or 60 mg/kg once every 2 weeks from confirmation of pregnancy at Gestation Day 20, throughout the gestation period, and continuing until 1‑month post-partum (approximately Lactation Day 28). There was no evidence of anifrolumab-fnia related maternal toxicity, embryo-fetal toxicity, or post-natal developmental effects. No anifrolumab-fnia related effect on T-cell-dependent antibody response in the infants was noted up to Day 180 after birth. The no observed adverse effect level (NOAEL) for maternal and developmental toxicity was identified as 60 mg/kg (approximately 28‑times the MRHD on an AUC basis). In the infants, mean serum concentrations of anifrolumab‑fnia on Day 30 after birth increased with dose and were approximately 4.2% to 9.7% of the respective maternal concentrations. The anifrolumab-fnia concentrations in the infant serum were up to approximately 22‑times the concentrations in the maternal milk, suggesting that anifrolumab-fnia had transferred via the placenta.
8.2 Lactation
Risk Summary
No data are available regarding the presence of SAPHNELO in human milk, the effects on the breastfed child, or the effects on milk production. Anifrolumab-fnia was detected in the milk of female cynomolgus monkeys administered anifrolumab-fnia. Due to species-species differences in lactation physiology, animal data may not reliably predict drug levels in humans. Maternal IgG is known to be present in human milk. If anifrolumab-fnia is transferred into human milk, the effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to anifrolumab-fnia are unknown.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for anifrolumab-fnia and any potential adverse effects on the breast-fed child from anifrolumab-fnia or from the underlying maternal condition.
-
11 DESCRIPTION
Anifrolumab-fnia is a type I interferon (IFN) receptor antagonist, immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that is produced in mouse myeloma cells (NS0) by recombinant DNA technology. The molecular weight is approximately 148 kDa.
SAPHNELO (anifrolumab-fnia) injection is a sterile, preservative‑free, clear to opalescent, colorless to slightly yellow, solution for intravenous use. SAPHNELO contains anifrolumab-fnia at a concentration of 150 mg/mL in a single-dose vial.
Each vial contains 300 mg (150 mg/mL) of anifrolumab-fnia, L-histidine (3 mg), L-histidine hydrochloride monohydrate (6 mg), L-lysine hydrochloride (18 mg), polysorbate 80 (1 mg), trehalose dihydrate (98 mg), and Water for Injection, USP. The pH is 5.9.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Anifrolumab-fnia is a human IgG1κ monoclonal antibody that binds to subunit 1 of the type I interferon receptor (IFNAR) with high specificity and affinity. This binding inhibits type I IFN signaling, thereby blocking the biologic activity of type I IFNs. Anifrolumab-fnia also induces the internalization of IFNAR1, thereby reducing the levels of cell surface IFNAR1 available for receptor assembly. Blockade of receptor mediated type I IFN signaling inhibits IFN responsive gene expression as well as downstream inflammatory and immunological processes. Inhibition of type I IFN blocks plasma cell differentiation and normalizes peripheral T-cell subsets.
Type I IFNs play a role in the pathogenesis of SLE. Approximately 60-80% of adult patients with active SLE express elevated levels of type I IFN inducible genes.
12.2 Pharmacodynamics
In patients with SLE, following the administration of anifrolumab-fnia at 300 mg dose, via intravenous infusion every 4 weeks for 52 weeks, neutralization (≥80%) of a type I IFN gene signature was observed from Week 4 to Week 52 in blood samples of patients with elevated levels of type I IFN inducible genes and returned to baseline levels within 8 to 12 weeks following withdrawal of anifrolumab-fnia at the end of the 52-week treatment period. However, the clinical relevance of the type I IFN gene signature neutralization is unclear.
In SLE patients with positive anti-dsDNA antibodies at baseline (Trials 2 and 3), treatment with anifrolumab-fnia 300 mg led to numerical reductions in anti-dsDNA antibodies over time through Week 52.
In patients with low complement levels (C3 and C4), increases in complement levels were observed in patients receiving anifrolumab-fnia through Week 52.
12.3 Pharmacokinetics
The PK of anifrolumab-fnia was studied in adult patients with SLE following intravenous doses ranging from 100 to 1000 mg once every 4 weeks, and healthy volunteers following a single intravenous dose at 300 mg. Anifrolumab-fnia exhibits non-linear PK in the dose range of 100 mg to 1000 mg with more than dose-proportional increases in the exposure as measured by AUC. Following the 300 mg every 4 weeks intravenous administrations of anifrolumab-fnia, steady-state was reached by Day 85. The accumulation ratio was approximately 1.36 for Cmax and 2.49 for Ctrough.
Distribution
Based on population PK analysis, the estimated volume of distribution at steady state for a typical patient with SLE (69.1 kg) is 6.23 L.
Elimination
From population PK analysis, anifrolumab-fnia exhibited non-linear PK due to IFNAR1-mediated drug clearance.
Following the administration of anifrolumab-fnia at a dose of 300 mg via intravenous infusion every 4 weeks, the estimated systemic clearance (CL) for anifrolumab-fnia was 0.193 L/day.
Based on population PK analysis of patients who received SAPHNELO for one year, serum concentrations of anifrolumab-fnia were below detection in 95% of patients approximately 16 weeks after the last dose.
Specific Populations
There was no clinically meaningful difference in systemic clearance based on age, race, ethnicity, region, gender, IFN status or body weight, that requires dose adjustment.
Age: Based on population PK analyses, age (range 18 to 69 years) did not affect anifrolumab-fnia clearance. Limited PK data are available for elderly patients; 3% (n=20) of the patients included in the PK analysis were 65 years or older [see Use in Specific Populations (8.5)].
Renal Impairment: No specific clinical studies have been conducted to investigate the effect of renal impairment on anifrolumab-fnia. Based on population PK analyses, anifrolumab-fnia clearance was comparable in SLE patients with mild (60-89 mL/min/1.73 m2) and moderate (30-59 mL/min/1.73 m2) decrease in eGFR values and patients with normal renal function (≥90 mL/min/1.73 m2). There were no SLE patients with a severe decrease in eGFR or end stage renal disease (<30 mL/min/1.73 m2); anifrolumab-fnia is not cleared renally.
Patients with UPCR >2 mg/mg were excluded from the clinical trials. Based on population PK analyses, increased urine protein/creatinine ratio (UPCR) did not significantly affect anifrolumab-fnia clearance.
Hepatic Impairment: No specific clinical studies have been conducted to investigate the effect of hepatic impairment on anifrolumab-fnia. IgG1 monoclonal antibodies are predominantly eliminated via catabolism and are not expected to undergo hepatic metabolism; changes in hepatic function are not expected to influence anifrolumab-fnia clearance. Based on population PK analyses, baseline hepatic function biomarkers (ALT and AST ≤2.0 × ULN, and total bilirubin) had no clinically relevant effect on anifrolumab-fnia clearance.
Drug Interactions
No formal drug-drug interaction studies have been conducted.
Based on population PK analysis, concomitant use of oral corticosteroids, anti-malarials, immunosuppressants (azathioprine, methotrexate, mycophenolate mofetil, mycophenolic acid, and mizoribine), NSAIDs, ACE inhibitors, and HMG-CoA reductase inhibitors did not significantly affect the PK of anifrolumab-fnia.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of anifrolumab-fnia.
In Trials 2, 3, and the long-term extension, treatment emergent anti-anifrolumab-fnia antibodies were detected in 9 of 350 (ADA incidence 2.6%) patients who received SAPHNELO at the recommended dosing regimen for up to 4 years.
Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of anifrolumab-fnia products is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic and genotoxic potential of anifrolumab-fnia have not been evaluated. In rodent models of IFNAR1 blockade, increased carcinogenic potential has been observed. The clinical relevance of these findings is unknown.
Effects on male and female fertility have not been directly evaluated in animal studies. No anifrolumab-fnia-related adverse effects on indirect measures of male or female fertility, based on semen analysis, spermatogenesis staging, menses cycle, organ weights and histopathological findings in the reproductive organs were observed in 9-month repeat-dose toxicity studies in cynomolgus monkeys at doses up to 50 mg/kg intravenous once weekly (approximately 58‑times the MRHD on an AUC basis).
-
14 CLINICAL STUDIES
The safety and efficacy of SAPHNELO were evaluated in three 52-week treatment period, multicenter, randomized, double-blind, placebo-controlled studies (Trial 1 [NCT01438489], Trial 2 [NCT02446912] and Trial 3 [NCT02446899]). Patients were diagnosed with SLE according to the American College of Rheumatology (1982 revised) classification criteria. All patients were ≥18 years of age and had moderate to severe disease, with a SLE Disease Activity Index 2000 (SLEDAI-2K) score ≥6 points, organ level involvement based on BILAG assessment, and a Physician’s Global Assessment [PGA] score ≥1, despite receiving standard SLE therapy consisting of either one or any combination of oral corticosteroids (OCS), antimalarials and/or immunosuppressants at baseline. Patients continued to receive their existing SLE therapy at stable doses during the clinical trials, with the exception of OCS (prednisone or equivalent) where tapering was a component of the protocol. Patients who had severe active lupus nephritis and patients who had severe active central nervous system lupus were excluded. The use of other biologic agents and cyclophosphamide were not permitted during the trials; patients receiving other biologic therapies were required to complete a wash-out period of at least 5 half‑lives prior to enrollment. All three studies were conducted in North America, Europe, South America and Asia. Patients received anifrolumab-fnia or placebo, administered by intravenous infusion, every 4 weeks.
Efficacy of SAPHNELO was established based on assessment of clinical response using the composite endpoints, the British Isles Lupus Assessment Group based Composite Lupus Assessment (BICLA) and the SLE Responder Index (SRI‑4).
BICLA response at Week 52, was defined as improvement in all organ domains with moderate or severe activity at baseline:
- •
- Reduction of all baseline BILAG A to B/C/D and baseline BILAG B to C/D, and no BILAG worsening in other organ systems, as defined by ≥1 new BILAG A or ≥2 new BILAG B;
- •
- No worsening from baseline in SLEDAI-2K, where worsening is defined as an increase from baseline of >0 points in SLEDAI-2K;
- •
- No worsening from baseline in patients’ lupus disease activity, where worsening is defined by an increase ≥0.30 points on a 3-point PGA VAS;
- •
- No discontinuation of treatment;
- •
- No use of restricted medication beyond the protocol-allowed threshold.
SRI‑4 response, was defined as meeting each of the following criteria at Week 52 compared with baseline:
- •
- Reduction from baseline of ≥4 points in the SLEDAI-2K;
- •
- No new organ system affected as defined by 1 or more BILAG A or 2 or more BILAG B items compared to baseline;
- •
- No worsening from baseline in the patients’ lupus disease activity defined by an increase ≥0.30 points on a 3‑point PGA visual analogue scale (VAS);
- •
- No discontinuation of treatment;
- •
- No use of restricted medication beyond the protocol-allowed threshold.
Trial 1 randomized 305 patients (1:1:1) who received anifrolumab-fnia, 300 mg or 1000 mg, or placebo for up to 52 weeks. The primary endpoint was a combined assessment of the SRI-4 and the sustained reduction in OCS (<10 mg/day and ≤OCS dose at week 1, sustained for 12 weeks) measured at Week 24.
Trial 2 and 3 were similar in design. Trial 2 randomized 457 patients who received anifrolumab-fnia 150 mg, 300 mg or placebo (1:2:2). Trial 3 randomized 362 patients (1:1) who received anifrolumab-fnia 300 mg or placebo. The primary endpoints were improvement in disease activity evaluated at 52 weeks, measured by SRI‑4 in Trial 2 and BICLA in Trial 3 (defined above). The common secondary efficacy endpoints included in both studies were the maintenance of OCS reduction, improvement in cutaneous SLE activity, and flare rate. During Weeks 8-40, patients with a baseline OCS ≥10 mg/day were required to taper their OCS dose to ≤7.5 mg/day, unless there was worsening of disease activity. Both studies evaluated the efficacy of anifrolumab-fnia 300 mg versus placebo; a dose of 150 mg was also evaluated for dose-response in Trial 2.
Patient demographics and disease characteristics were generally similar and balanced across treatment arms (Table 2).
Table 2 Demographics and Baseline Characteristics Total Population
Trial 1
(N = 305)
Trial 2
(N = 457)
Trial 3
(N = 362)
Mean Age (years)
40
41
42
Female (%)
93
92
93
White (%)
42
71
60
Black/African American (%)
13
14
12
Asian (%)
7
5
17
Hispanic or Latino (%)
42
19
30
Baseline SLEDAI-2K score
Mean (SD)
10.9 (4.1)
11.3 (3.72)
11.5 (3.76)
≥10 points, n (%)
182 (60)
328 (72)
260 (72)
BILAG organ system scoring (Overall)
At least one A, n (%)
152 (50)
217 (48)
176 (49)
No A and at least 2 Bs, n (%)
134 (44)
211 (46)
169 (47)
Positive Anti-dsDNA levels, n (%)
185 (77)
207 (45)
159 (44)
Abnormal ANA, n (%)
299 (98)
412 (90)
325 (90)
Abnormal Complement C3 level, n (%)
119 (39)
157 (34)
144 (40)
Abnormal Complement C4 level, n (%)
74 (24)
95 (21)
95 (26)
Baseline SLE treatment
OCS, n (%)
258 (85)
381 (83)
292 (81)
Antimalarials, n (%)
219 (72)
334 (73)
252 (70)
Immunosuppressants, n (%)
150 (49)
214 (47)
174 (48)
Randomization was stratified by disease severity (SLEDAI-2K score at baseline, <10 vs ≥10 points), OCS dose on Day 1 (<10 mg/day vs ≥10 mg/day prednisone or equivalent) and interferon gene signature test results (high vs low).
The reduction in disease activity seen in the BICLA and SRI-4 was related primarily to improvement in the mucocutaneous and musculoskeletal organ systems. Flare rate was reduced in patients receiving SAPHNELO compared to patients who received placebo although the difference was not statistically significant.
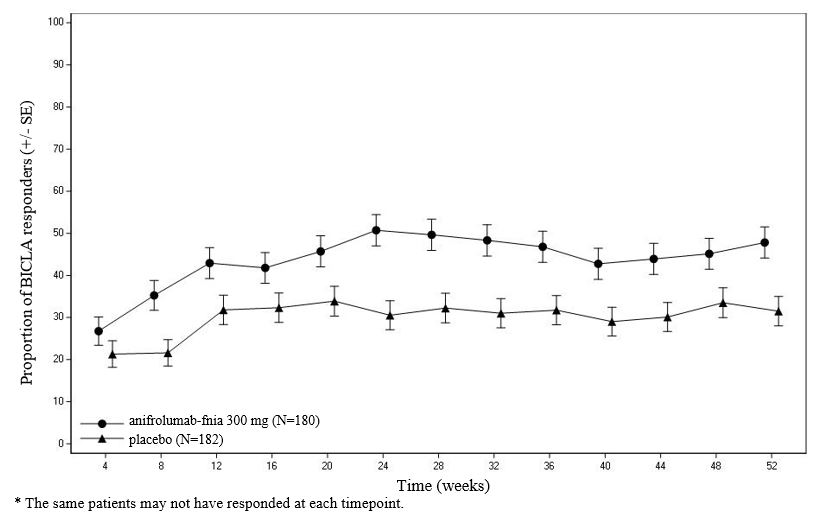
BICLA responder analysis: BICLA was the primary endpoint in Trial 3; anifrolumab-fnia 300 mg demonstrated statistically significant and clinically meaningful efficacy in overall disease activity compared with placebo, with greater improvements in all components of the composite endpoint. In Trial 1 and 2 BICLA was a pre-specified analysis. The BICLA results are presented in Table 3.
Table 3 BICLA Response Rate at Week 52 - *
- Not formally tested in a pre-specified testing scheme and findings should be interpreted with caution.
- †
- Based on post hoc analysis.
- ‡
- Primary endpoint.
- §
- In all 3 trials, patients who discontinued investigational product or initiated restricted medications beyond the protocol-specified thresholds are considered non-responders. For consistency, the results presented for Trial 2 represent the post-hoc analysis using the restricted medication thresholds as defined in Trial 3.
Trial 3‡
Anifrolumab- fnia 300 mg
(N=99)
Placebo
(N=102)
Anifrolumab- fnia 300 mg
(N=180)
Placebo
(N=184)
Anifrolumab- fnia 300 mg
(N=180)
Placebo
(N=182)
BICLA Response Rate§
Responder, n (%)
54 (54.6)
27 (25.8)
85 (47.1)
55 (30.2)
86 (47.8)
57 (31.5)
Difference in Response Rates (95% CI)
28.8 (15.7, 41.9)
17.0 (7.2, 26.8)
16.3 (6.3, 26.3)
p-value = 0.001
Components of BICLA Response§
BILAG Improvement, n (%)
54 (54.5)
28 (27.5)
85 (47.2)
58 (31.5)
88 (48.9)
59 (32.4)
No Worsening of SLEDAI-2K, n (%)
73 (73.7)
61 (59.8)
121 (67.2)
104 (56.5)
122 (67.8)
94 (51.6)
No Worsening of PGA, n (%)
76 (76.8)
62 (60.8)
117 (65.0)
105 (57.1)
122 (67.8)
95 (52.2)
The response rates and associated difference and 95% CI are calculated using a Cochran-Mantel-Haenszel approach adjusted for stratification factors. The reported percentages for the components are unadjusted.
In Trial 3, examination of subgroups by age, race, gender, ethnicity, disease severity [SLEDAI-2K at baseline], and baseline OCS use did not identify differences in response to anifrolumab-fnia.
Figure 1 shows the proportion of BICLA responders through the 52-week treatment period in Trial 3.
Figure 1 Trial 3: Proportion (%) of BICLA Responders by Visit*
SRI-4 responder analysis: SRI-4 was the primary endpoint in Trial 2; treatment with anifrolumab-fnia did not result in statistically significant improvements over placebo. In Trials 1 and 3, SRI-4 was a pre-specified analysis. The SRI-4 results are presented in Table 4.
Table 4 SRI-4 Response Rate at Week 52 - *
- Not formally tested in a pre-specified testing scheme and findings should be interpreted with caution.
- †
- Primary endpoint.
- ‡
- In all 3 studies, patients who discontinued investigational product or initiated restricted medications beyond the protocol-specified thresholds are considered non-responders. For consistency, the results presented for Trial 2 represent the post-hoc analysis using the restricted medication thresholds as defined in Trial 3. The most commonly involved SLEDAI-2K organ domains were mucocutaneous, musculoskeletal and immune.
Trial 1*
Trial 2†
Trial 3*
Anifrolumab- fnia 300 mg
(N=99)
Placebo
(N=102)
Anifrolumab- fnia 300 mg
(N=180)
Placebo
(N=184)
Anifrolumab- fnia 300 mg
(N=180)
Placebo
(N=182)
SRI-4 Response Rate‡
Responder, n (%)
62 (62.8)
41 (38.8)
88 (49.0)
79 (43.0)
100 (55.5)
68 (37.3)
Difference in Response Rates (95% CI)
24.0 (10.9, 37.2)
6.0 (-4.2, 16.2)
18.2 (8.1, 28.3)
Components of SRI-4 Response‡
SLEDAI-2K improvement, n (%)
62 (62.6)
41 (40.2)
89 (49.4)
80 (43.5)
101 (56.1)
71 (39.0)
No worsening of BILAG, n (%)
75 (75.8)
61 (59.8)
119 (66.1)
105 (57.1)
125 (69.4)
94 (51.6)
No worsening of PGA, n (%)
76 (76.8)
62 (60.8)
117 (65.0)
105 (57.1)
122 (67.8)
95 (52.2)
The response rates and associated difference and 95% CI are calculated using a Cochran-Mantel-Haenszel approach adjusted for stratification factors. The reported percentages for the components are unadjusted.
Effect on Concomitant Steroid Treatment: In Trial 3, among the 47% of patients with a baseline OCS use ≥10 mg/day, anifrolumab-fnia demonstrated a statistically significant difference in the proportion of patients able to reduce OCS use by at least 25% to ≤7.5 mg/day at Week 40 and maintain the reduction through Week 52 (p-value = 0.004); 52% (45/87) of patients in the anifrolumab-fnia group versus 30% (25/83) in the placebo achieved this level of steroid reduction (difference 21% [95% CI 6.8, 35.7]). Consistent trends in favor of anifrolumab-fnia compared to placebo, on effect of reduction of OCS use, were observed in Trial 1 and 2, but the difference was not statistically significant.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
SAPHNELO (anifrolumab-fnia) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for intravenous infusion. It is packaged in a 2 mL clear glass vial containing 300 mg/2 mL (150 mg/mL) of anifrolumab-fnia.
SAPHNELO is available in a carton containing one single-dose vial (NDC-0310-3040-00).
Store in a refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
Do not freeze. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Serious Infections
Inform patients that SAPHNELO may decrease their ability to fight infections, and that serious infections, including fatal ones, occurred in patients receiving SAPHNELO in clinical trials. Also inform patients that they are at increased risk of respiratory infections and herpes zoster during treatment with SAPHNELO [see Warnings and Precautions (5.1)]. Advise patients to contact their healthcare provider if they develop any symptoms of an infection, including fever or flu-like symptoms; muscle aches; cough; shortness of breath; burning when they urinate or urinating more often than usual; diarrhea or stomach pain; shingles (a red skin rash that can cause pain and burning).
Hypersensitivity Reactions/Anaphylaxis
Inform patients that serious hypersensitivity reactions, including anaphylaxis, have been reported in patients who received SAPHNELO. Instruct patients to immediately tell their healthcare provider or go to the emergency department of their nearest hospital, if they experience symptoms of an allergic reaction (e.g., anaphylaxis) during or after the administration of SAPHNELO [see Warnings and Precautions (5.2)]. Symptoms may include swelling of the face, tongue, or mouth, breathing difficulties, and/or fainting, dizziness, feeling lightheaded (due to a drop in blood pressure).
Immunizations
Inform patients that they should not receive live or live-attenuated vaccines while receiving SAPHNELO. Advise patients to discuss with their healthcare provider before seeking immunizations on their own [see Warnings and Precautions (5.4)].
Pregnancy
Advise female patients to inform their healthcare provider if they intend to become pregnant during therapy, suspect they are pregnant or become pregnant while receiving SAPHNELO [see Use in Specific Populations (8.1)].
Inform women that they can find information about a pregnancy exposure registry which monitors pregnancy outcomes in women exposed to SAPHNELO by calling AstraZeneca at 1-877-693-9268.
Manufactured by: AstraZeneca AB Södertälje, Sweden SE-15185
US License No. 2059
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2024
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
SAPHNELO® (saf-NEH-low)
(anifrolumab-fnia)
injection, for intravenous use
What is SAPHNELO?
- •
- SAPHNELO is a prescription medicine used to treat adults with moderate to severe systemic lupus erythematosus (SLE or lupus) who are receiving other lupus medicines.
- •
- SAPHNELO contains anifrolumab-fnia which is in a group of medicines called monoclonal antibodies. Lupus is a disease of the immune system (the body system that fights infection). When given together with other medicines for lupus, SAPHNELO may help to reduce your lupus disease activity more than other lupus medicines alone.
- •
- It is not known if SAPHNELO is effective in people with severe active lupus nephritis or central nervous system lupus.
- •
- It is not known if SAPHNELO is safe and effective in children under 18 years of age.
Do not use SAPHNELO if you:
- •
- are allergic to anifrolumab-fnia or any of the ingredients in SAPHNELO. See the end of this Patient Information leaflet for a complete list of ingredients in SAPHNELO.
Before you receive SAPHNELO, tell your healthcare provider about all of your medical conditions, including if you:
- •
- think you have an infection or have infections that keep coming back. You should not receive SAPHNELO if you have an infection unless your healthcare provider tells you to. See “What are the possible side effects of SAPHNELO?”
- •
- are scheduled to receive a vaccination or if you think you may need a vaccination. You should not receive live vaccines during treatment with SAPHNELO.
- •
- have or have had any type of cancer.
- •
- are receiving other biologic medicines or monoclonal antibodies.
- •
- are pregnant or plan to become pregnant. It is not known if SAPHNELO will harm your unborn baby. Tell your healthcare provider if you are pregnant, think you might be pregnant, or plan to become pregnant during your treatment with SAPHNELO.
- ∘
- Pregnancy Exposure Registry. If you become pregnant while receiving SAPHNELO, talk to your healthcare provider. A pregnancy exposure registry monitors pregnancy outcomes in women exposed to SAPHNELO. You can find out more information about the registry by calling AstraZeneca at 1-877-693-9268.
- •
- are breastfeeding or plan to breastfeed. It is not known if SAPHNELO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while receiving SAPHNELO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. SAPHNELO may affect the way other medicines work, and other medicines may affect how SAPHNELO works.
How will I receive SAPHNELO?
- •
- Your healthcare provider will give you SAPHNELO through a needle placed in a vein (IV or intravenous infusion). It takes about 30 minutes to give you the full dose of SAPHNELO.
- •
- SAPHNELO is usually given 1 time every 4 weeks.
- •
- If you miss an appointment, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of SAPHNELO?
SAPHNELO may cause serious side effects, including:
- •
- Serious Infections. SAPHNELO can lower the ability of your immune system to fight infections. You may be at a higher risk of developing respiratory infections, and shingles (herpes zoster) during treatment with SAPHNELO. Infections (including COVID-19) could be serious, leading to hospitalization or death. Tell your healthcare provider right away if you have any of the following symptoms of an infection:
- •
- fever, sweating, or chills
- •
- muscle aches
- •
- cough
- •
- shortness of breath
- •
- burning when urinating
- •
- urinating more often
- •
- diarrhea or stomach pain
- •
- warm, red, or painful skin or sores on your body.
- •
- Allergic (hypersensitivity) reactions, including anaphylaxis. Serious allergic reactions can happen during or after you get your SAPHNELO infusion. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of a serious allergic reaction:
- •
- swelling of your face, mouth, and tongue
- •
- breathing problems
- •
- fainting or dizziness
- •
- feeling lightheaded (low blood pressure)
- •
- Cancer. SAPHNELO may reduce the activity of your immune system. Medicines that affect the immune system may increase your risk of certain cancers.
The most common side effects of SAPHNELO include:
-
- •
- upper respiratory infections
- •
- infusion reactions
- •
- cough
- •
- bronchitis
- •
- shingles (herpes zoster)
These are not all of the possible side effects of SAPHNELO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of SAPHNELO
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information about SAPHNELO, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about SAPHNELO that is written for health professionals.
What are the ingredients in SAPHNELO?
Active ingredient: anifrolumab-fnia
Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, L-lysine hydrochloride, trehalose dihydrate, polysorbate 80 and Water for Injection.
Manufactured by: AstraZeneca AB Södertälje, Sweden SE-15185
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2023
For more information, go to https://www.SAPHNELO.com or call 1-800-236-9933.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: December 2023
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 300mg/2mL (150 mg/mL)
NDC 0310-3040-00 Rx only
SAPHNELO®
(anifrolumab-fnia)
Injection
300 mg/2 mL
(150 mg/mL)
For Intravenous Infusion After Dilution
Must dilute before use. See prescribing information.
Single-dose vial. Discard unused portion.
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Keep vial in original carton to protect from light.
Do not shake or freeze.
1 single-dose vial
AstraZeneca
-
INGREDIENTS AND APPEARANCE
SAPHNELO
anifrolumab-fnia injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-3040 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANIFROLUMAB (UNII: 38RL9AE51Q) (ANIFROLUMAB - UNII:38RL9AE51Q) ANIFROLUMAB 300 mg in 2.0 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 3 mg in 2.0 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 6 mg in 2.0 mL LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) 18 mg in 2.0 mL TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 98 mg in 2.0 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 mg in 2.0 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-3040-00 1 in 1 CARTON 07/30/2021 1 2.0 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761123 07/30/2021 Labeler - AstraZeneca Pharmaceuticals LP (054743190)