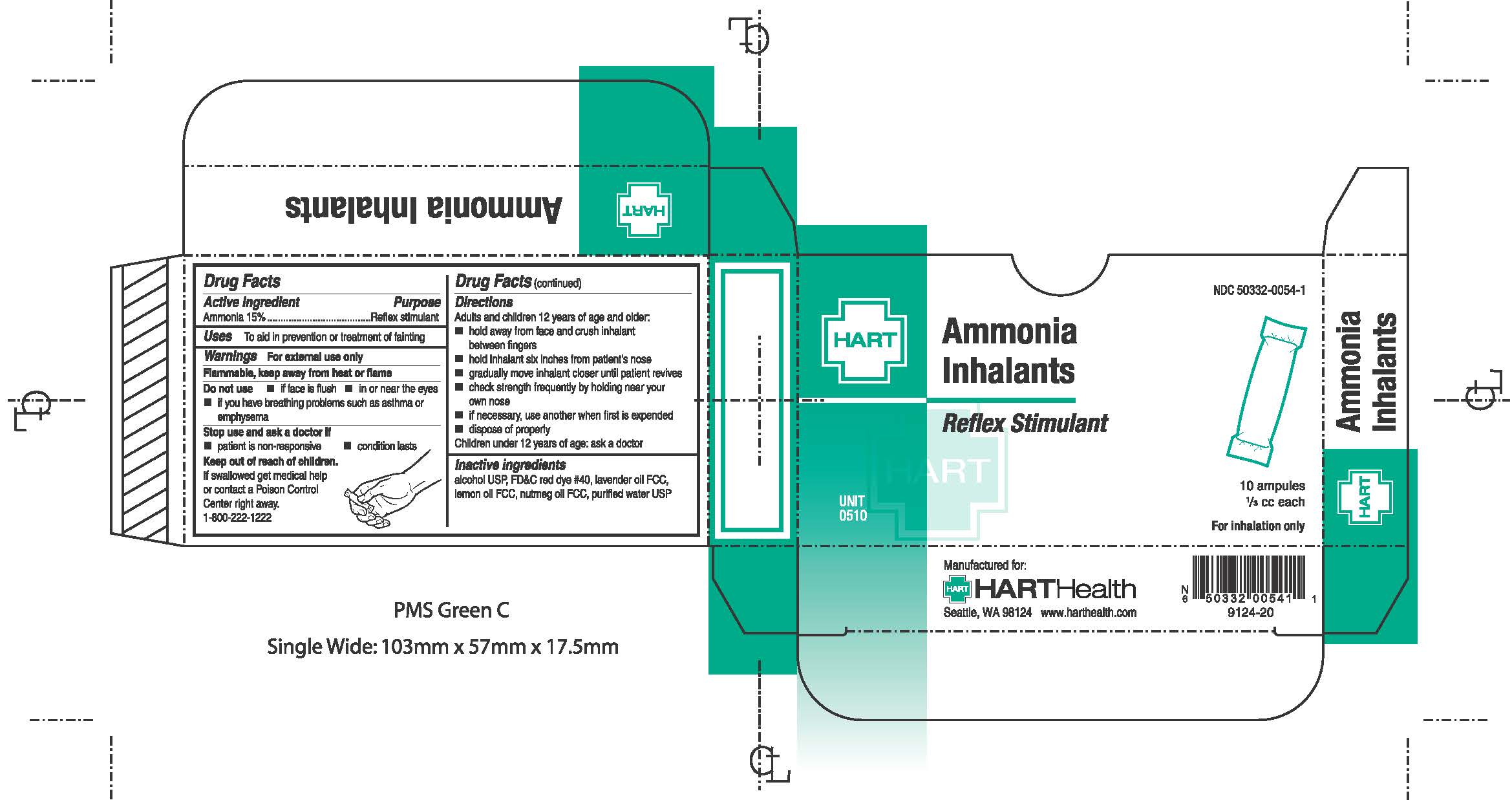
Label: AMMONIA INHALANTS- ammonia inhalant
-
Contains inactivated NDC Code(s)
NDC Code(s): 50332-0054-1 - Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 4, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children 12 years of age and older:
- hold away from face and crush inhalant between fingers
- hold packet six inches from patient's nose
- gradually move inhalant closer until patient revives
- check strength frequently by holding near your own nose
- if necessary, use another when first is expended
- dispose of properly
Children under 12 years of age: ask a doctor
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMMONIA INHALANTS
ammonia inhalantProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0054 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.15 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) FD&C RED NO. 40 (UNII: WZB9127XOA) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0054-1 10 in 1 BOX 01/04/2021 1 .15 g in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2000 Labeler - HART Health (069560969)