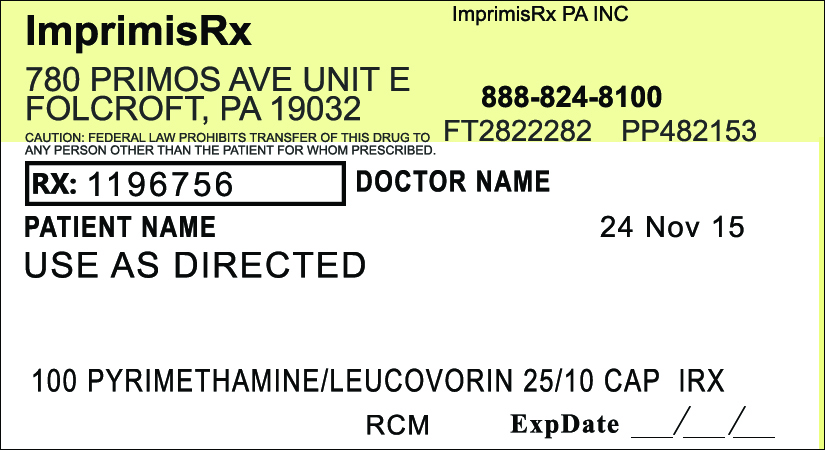
Label: PYRIMETHAMINE LEUCOVORIN capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 70271-427-01 - Packager: ImprimisRx PA, Inc. d/b/a ImprimisRx
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 27, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Pyrimethamine Leucovorin (25mg-10mg)
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
PYRIMETHAMINE LEUCOVORIN
pyrimethamine leucovorin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70271-427 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIMETHAMINE (UNII: Z3614QOX8W) (PYRIMETHAMINE - UNII:Z3614QOX8W) PYRIMETHAMINE 25 mg LEUCOVORIN CALCIUM (UNII: RPR1R4C0P4) (LEUCOVORIN - UNII:Q573I9DVLP) LEUCOVORIN 10 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 85 mg Product Characteristics Color pink, yellow Score no score Shape capsule Size 15mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70271-427-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/27/2015 Labeler - ImprimisRx PA, Inc. d/b/a ImprimisRx (080044737)