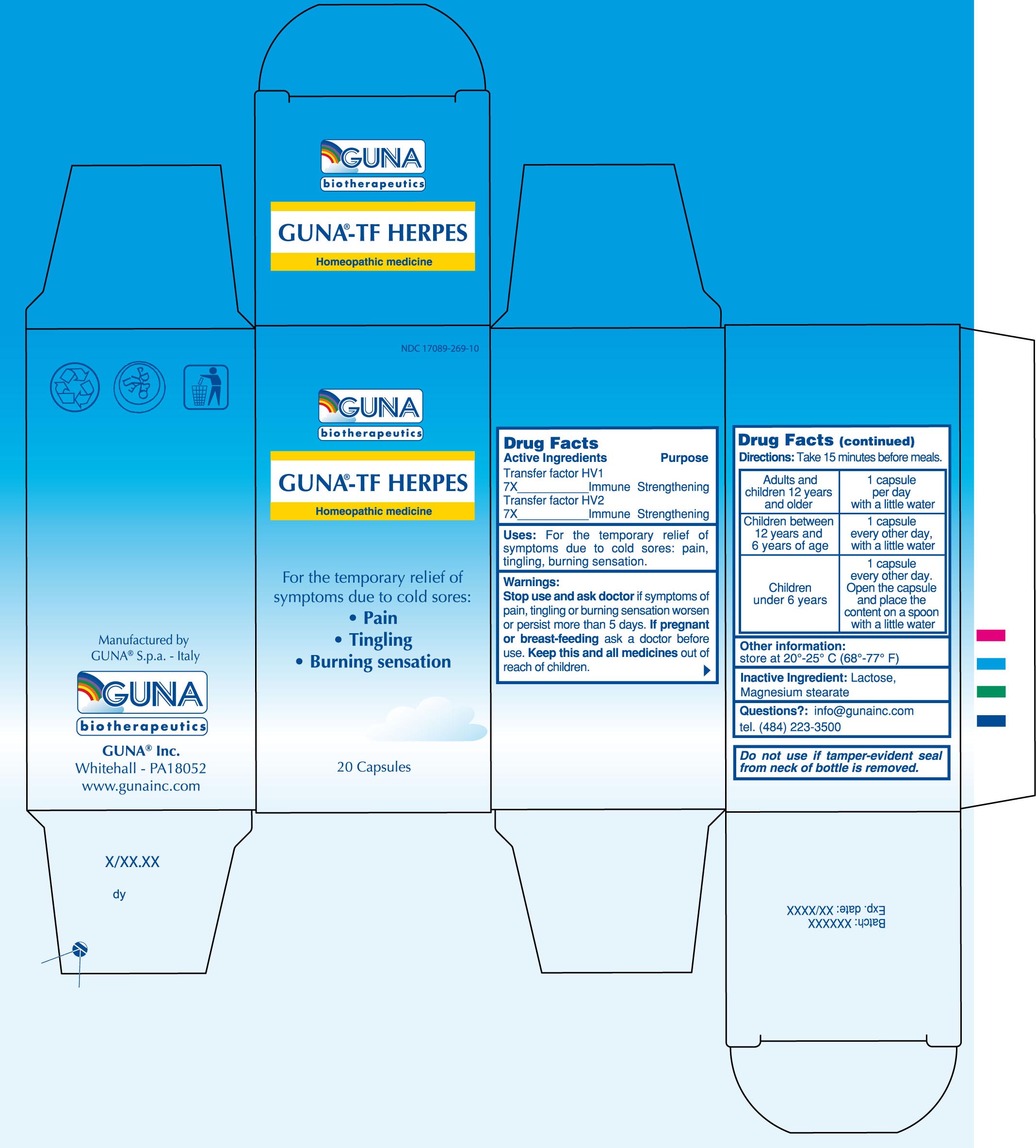
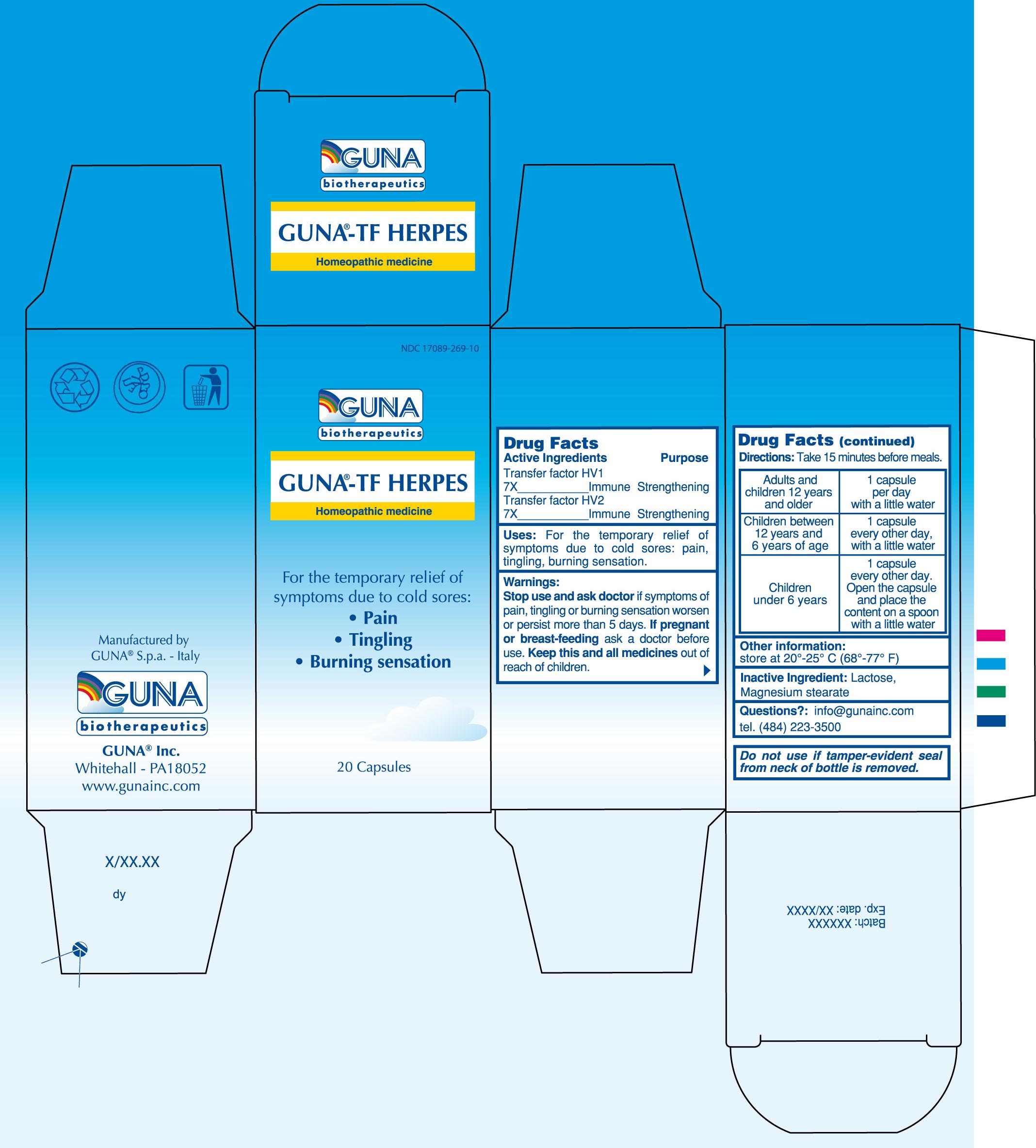
Label: GUNA-TF HERPES- human herpesvirus 1 - human herpesvirus 2 - capsule, gelatin coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 17089-269-10 - Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 31, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS/PURPOSE
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
-
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 1 capsule per day with a little water
Children between 12 years and 6 years of age 1 capsule every other day, with a little water
Children under 6 years 1 capsule every other day. Open the capsule and place the content on a spoon with a little water
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-TF HERPES
human herpesvirus 1 - human herpesvirus 2 - capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-269 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN HERPESVIRUS 1 (UNII: 22G38P19RL) (HUMAN HERPESVIRUS 1 - UNII:22G38P19RL) HUMAN HERPESVIRUS 1 7 [hp_X] in 4600 mg HUMAN HERPESVIRUS 2 (UNII: 74J6DNH49U) (HUMAN HERPESVIRUS 2 - UNII:74J6DNH49U) HUMAN HERPESVIRUS 2 7 [hp_X] in 4600 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) 4048 mg in 4600 mg MAGNESIUM STEARATE (UNII: 70097M6I30) 92 mg in 4600 mg Product Characteristics Color white (white) Score 2 pieces Shape OVAL (Capsule) Size 18mm Flavor Imprint Code na Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-269-10 1 in 1 BOX 1 4600 mg in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 430538264 manufacture