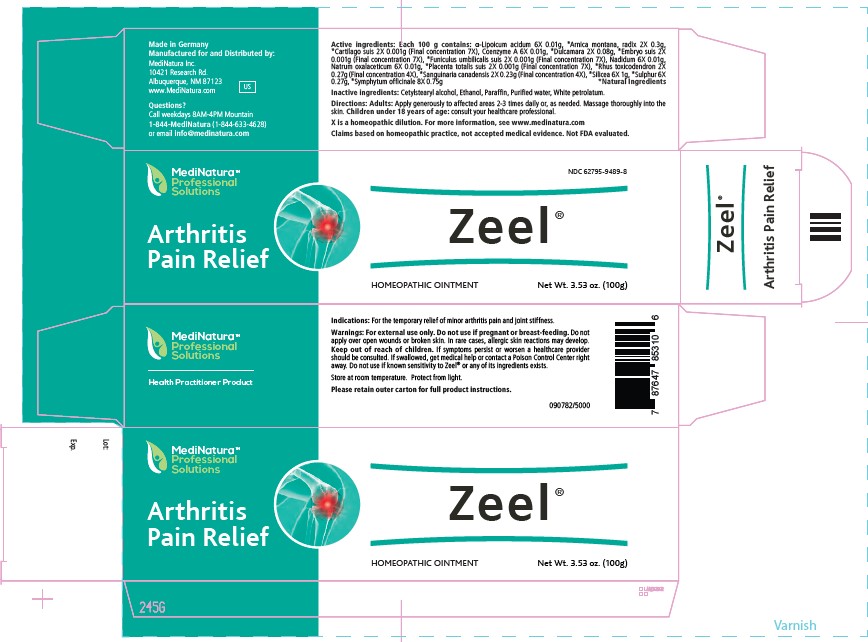
Label: ZEEL- alpha lipoic acid, arnica montana whole,sus scrofa cartilage, coenzyme a,solanum dulcamara top,sus scrofa embryo,sus scrofa umbilical cord, nadide, sodium diethyl oxalacetate,sus scrofa placenta,toxicodendron pubescens leaf, sanguinaria canadensis root, silicon dioxide,sulfur,coriander ointment
- NDC Code(s): 51885-9489-8
- Packager: Biologische Heilmittel Heel
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Warnings
Warnings: For external use only. Do not use if
pregnant or breast-feeding. Do not apply over open
wounds or broken skin. In rare cases, allergic skin reactions
may develop. Keep out of reach of children. If
symptoms persist or worsen a healthcare provider should be
consulted. If swallowed, get medical help or contact a
Poison Control Center right away. Do not use if known
sensitivity to Zeel® or any of its ingredients exists. - Directions
- Inactive ingredients:
- KEEP OUT OF REACH OF CHILDREN
- Indications
- Purpose
-
Active Ingrendent
Active ingredients: Each 100 g contains: _-Lipoicum acidum
6X 0.01g, *Arnica montana, radix 2X 0.3g, *Cartilago suis 2X
0.001g (Final concentration 7X), Coenzyme A 6X 0.01g, *Dulcamara
2X 0.08g, *Embryo suis 2X 0.001g (Final concentration 7X),
*Funiculus umbilicalis suis 2X 0.001g (Final concentration 7X)
Nadidum 6X 0.01g, Natrum oxalaceticum 6X 0.01g, *Placenta
totalis suis 2X 0.001g (Final concentration 7X), *Rhus toxicodendron
2X 0.27g (Final concentration 4X), *Sanguinaria canadensis 2X
0.23g (Final concentration 4X), *Silicea 6X 1g, *Sulphur 6X 0.27g,
*Symphytum officinale 8X 0.75g. *Natural Ingredient - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZEEL
alpha lipoic acid, arnica montana whole,sus scrofa cartilage, coenzyme a,solanum dulcamara top,sus scrofa embryo,sus scrofa umbilical cord, nadide, sodium diethyl oxalacetate,sus scrofa placenta,toxicodendron pubescens leaf, sanguinaria canadensis root, silicon dioxide,sulfur,coriander ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51885-9489 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPHA LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) ALPHA LIPOIC ACID 6 [hp_X] in 1 g ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 2 [hp_X] in 1 g SUS SCROFA CARTILAGE (UNII: 73ECW5WG2F) (SUS SCROFA CARTILAGE - UNII:73ECW5WG2F) SUS SCROFA CARTILAGE 2 [hp_X] in 1 g COENZYME A (UNII: SAA04E81UX) (COENZYME A - UNII:SAA04E81UX) COENZYME A 6 [hp_X] in 1 g SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 2 [hp_X] in 1 g SUS SCROFA EMBRYO (UNII: 9928MC12VO) (SUS SCROFA EMBRYO - UNII:9928MC12VO) SUS SCROFA EMBRYO 2 [hp_X] in 1 g SUS SCROFA UMBILICAL CORD (UNII: 118OYG6W3H) (SUS SCROFA UMBILICAL CORD - UNII:118OYG6W3H) SUS SCROFA UMBILICAL CORD 2 [hp_X] in 1 g NADIDE (UNII: 0U46U6E8UK) (NADIDE - UNII:0U46U6E8UK) NADIDE 6 [hp_X] in 1 g SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 6 [hp_X] in 1 g SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 2 [hp_X] in 1 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 2 [hp_X] in 1 g SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 2 [hp_X] in 1 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 6 [hp_X] in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] in 1 g CORIANDER (UNII: 1OV56052IK) (CORIANDER - UNII:1OV56052IK) CORIANDER 8 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ALCOHOL (UNII: 3K9958V90M) PARAFFIN (UNII: I9O0E3H2ZE) WHITE PETROLATUM (UNII: B6E5W8RQJ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51885-9489-8 1 in 1 CARTON 09/22/2021 1 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2021 Labeler - Biologische Heilmittel Heel (315635359) Establishment Name Address ID/FEI Business Operations Biologische Heilmittel Heel 315635359 manufacture(51885-9489)