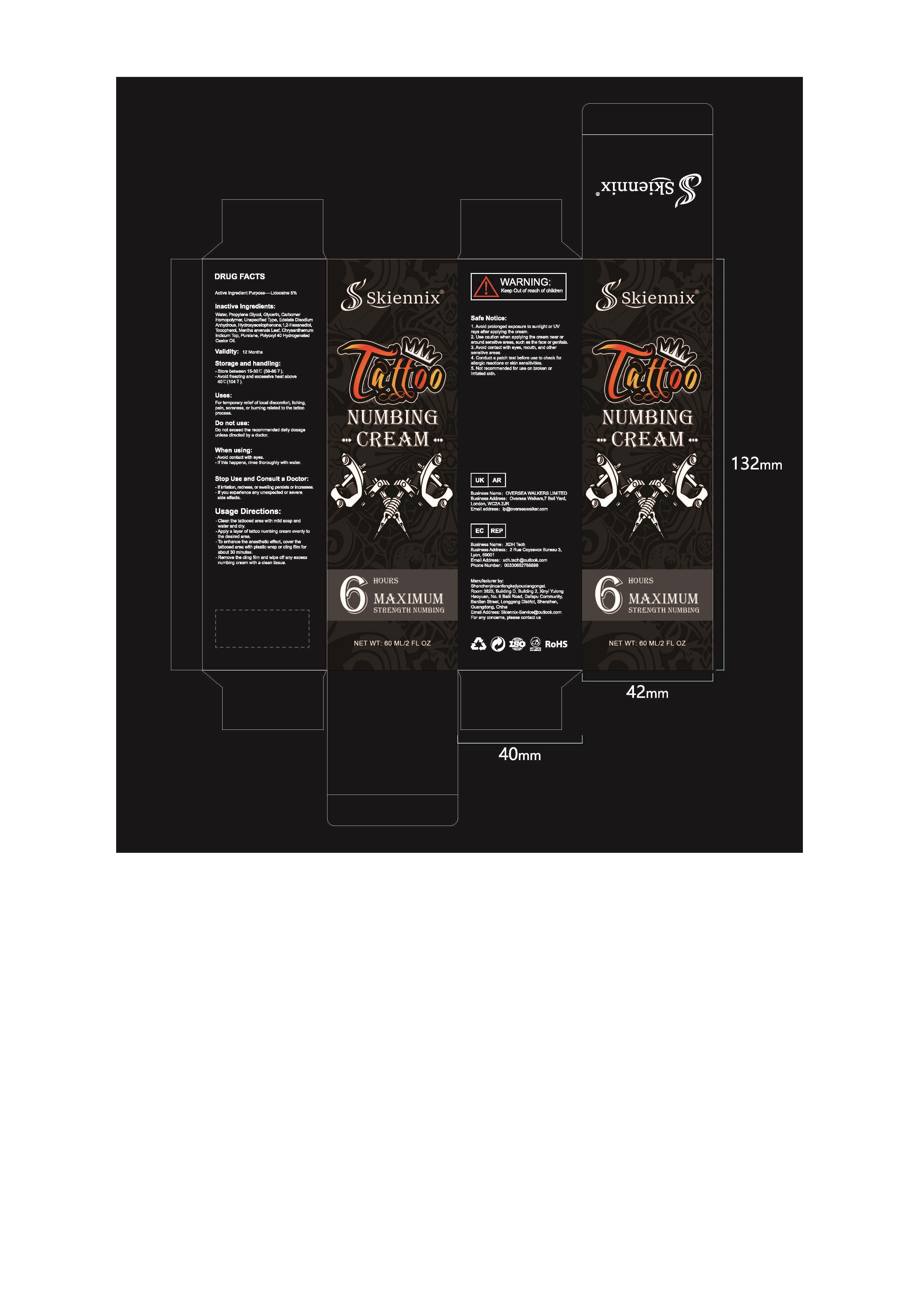
Label: TATTOO NUMBING- lidocaine cream
- NDC Code(s): 84787-001-02
- Packager: Shenzhen NuoYiyan Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Safe Notice
- Do not use
- When using
- Stop Use and Consult a Doctor
- KEEP OUT OF REACH OF CHILDREN
-
USAGE DIRECTIONS
1. Clean the tattooed area with mild soap and water and dry.
2.Apply a layer of tattoo numbing cream evenly to the desired area.
3. To enhance the anesthetic effect, cover the tattooed area with plastic wrapor cling film for about 30 minutes.
4. Remove the cling film and wipe off any excess numbing cream with a cleantissue.
- Storage and handling
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TATTOO NUMBING
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84787-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) Lidocaine 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TOCOPHEROL (UNII: R0ZB2556P8) MENTHA ARVENSIS LEAF (UNII: A4IWO4DDZ9) CHRYSANTHELLUM INDICUM TOP (UNII: STJ856D1Z0) PURSLANE (UNII: M6S840WXG5) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84787-001-02 1 in 1 BOX 10/08/2024 1 60 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 10/08/2024 Labeler - Shenzhen NuoYiyan Technology Co., Ltd. (700126426) Registrant - Shenzhen NuoYiyan Technology Co., Ltd. (700126426) Establishment Name Address ID/FEI Business Operations Shenzhen NuoYiyan Technology Co., Ltd. 700126426 manufacture(84787-001)