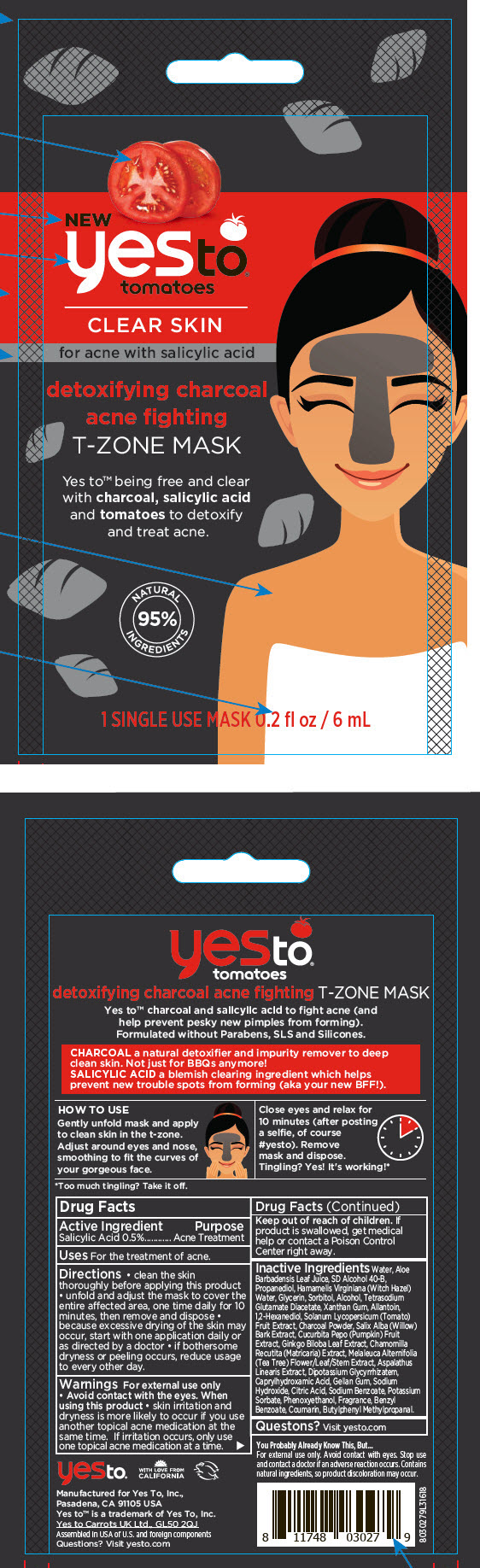
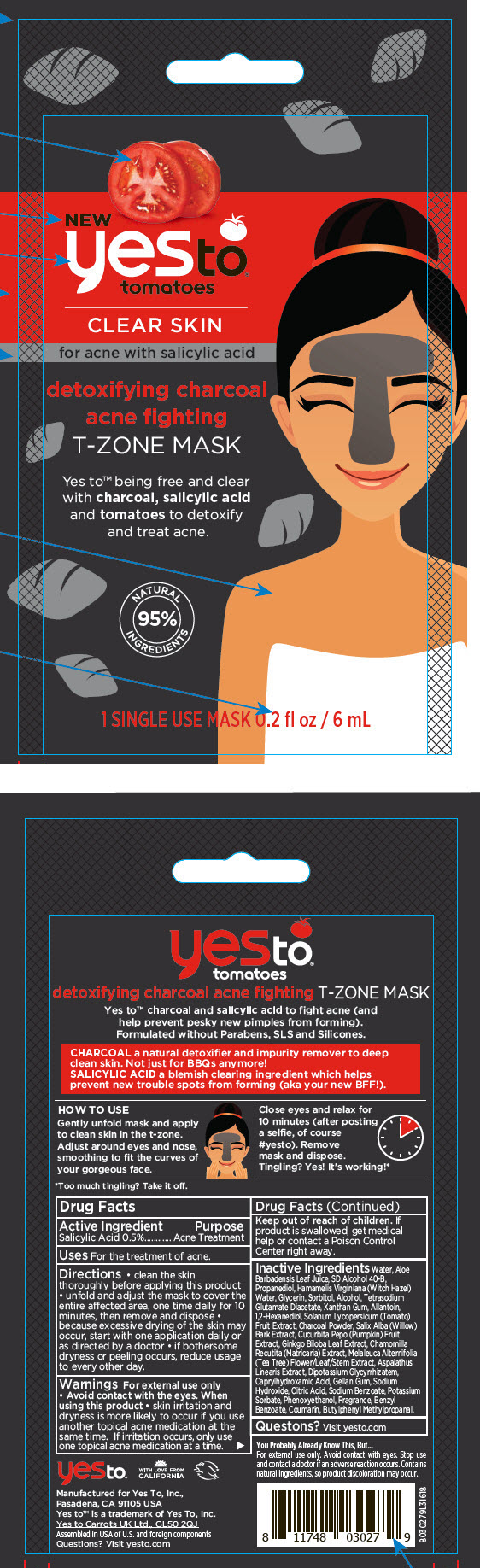
Label: YES TO TOMATOES DETOXIFYING CHARCOAL ACNE FIGHTING T-ZONE MASK- salicylic acid strip
-
Contains inactivated NDC Code(s)
NDC Code(s): 69840-012-03 - Packager: Yes To, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Directions
- clean the skin thoroughly before applying this product
- unfold and adjust the mask to cover the entire affected area, one time daily for 10 minutes, then remove and dispose
- because excessive drying of the skin may occur, start with one application daily or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce usage to every other day.
- Warnings
-
Inactive Ingredients
Water, Aloe Barbadensis Leaf Juice, SD Alcohol 40-B, Propanediol, Hamamelis Virginiana (Witch Hazel) Water, Glycerin, Sorbitol, Alcohol, Tetrasodium Glutamate Diacetate, Xanthan Gum, Allantoin, 1,2-Hexanediol, Solanum Lycopersicum (Tomato) Fruit Extract, Charcoal Powder, Salix Alba (Willow) Bark Extract, Cucurbita Pepo (Pumpkin) Fruit Extract, Ginkgo Biloba Leaf Extract, Chamomilla Recutita (Matricaria) Extract, Melaleuca Alternifolia (Tea Tree) Flower/Leaf/Stem Extract, Aspalathus Linearis Extract, Dipotassium Glycyrrhizatem, Caprylhydroxamic Acid, Gellan Gum, Sodium Hydroxide, Citric Acid, Sodium Benzoate, Potassium Sorbate, Phenoxyethanol, Fragrance, Benzyl Benzoate, Coumarin, Butylphenyl Methylpropanal.
- Questons?
- PRINCIPAL DISPLAY PANEL - 1 Patch Pouch
-
INGREDIENTS AND APPEARANCE
YES TO TOMATOES DETOXIFYING CHARCOAL ACNE FIGHTING T-ZONE MASK
salicylic acid stripProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69840-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) PROPANEDIOL (UNII: 5965N8W85T) ALLANTOIN (UNII: 344S277G0Z) GLYCERIN (UNII: PDC6A3C0OX) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) SOLANUM LYCOPERSICUM (UNII: 0243Q4990L) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) SORBITOL (UNII: 506T60A25R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) ENOXOLONE DIPOTASSIUM (UNII: ZJI2YIM6Z9) XANTHAN GUM (UNII: TTV12P4NEE) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) GELLAN GUM (LOW ACYL) (UNII: 7593U09I4D) SALIX ALBA BARK (UNII: 205MXS71H7) GINKGO (UNII: 19FUJ2C58T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PUMPKIN (UNII: SYW0QUB89Y) MATRICARIA CHAMOMILLA LEAF (UNII: 6I9LN466F0) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69840-012-03 1 in 1 POUCH 01/01/2019 1 6 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 01/01/2019 Labeler - Yes To, Inc. (788689680)