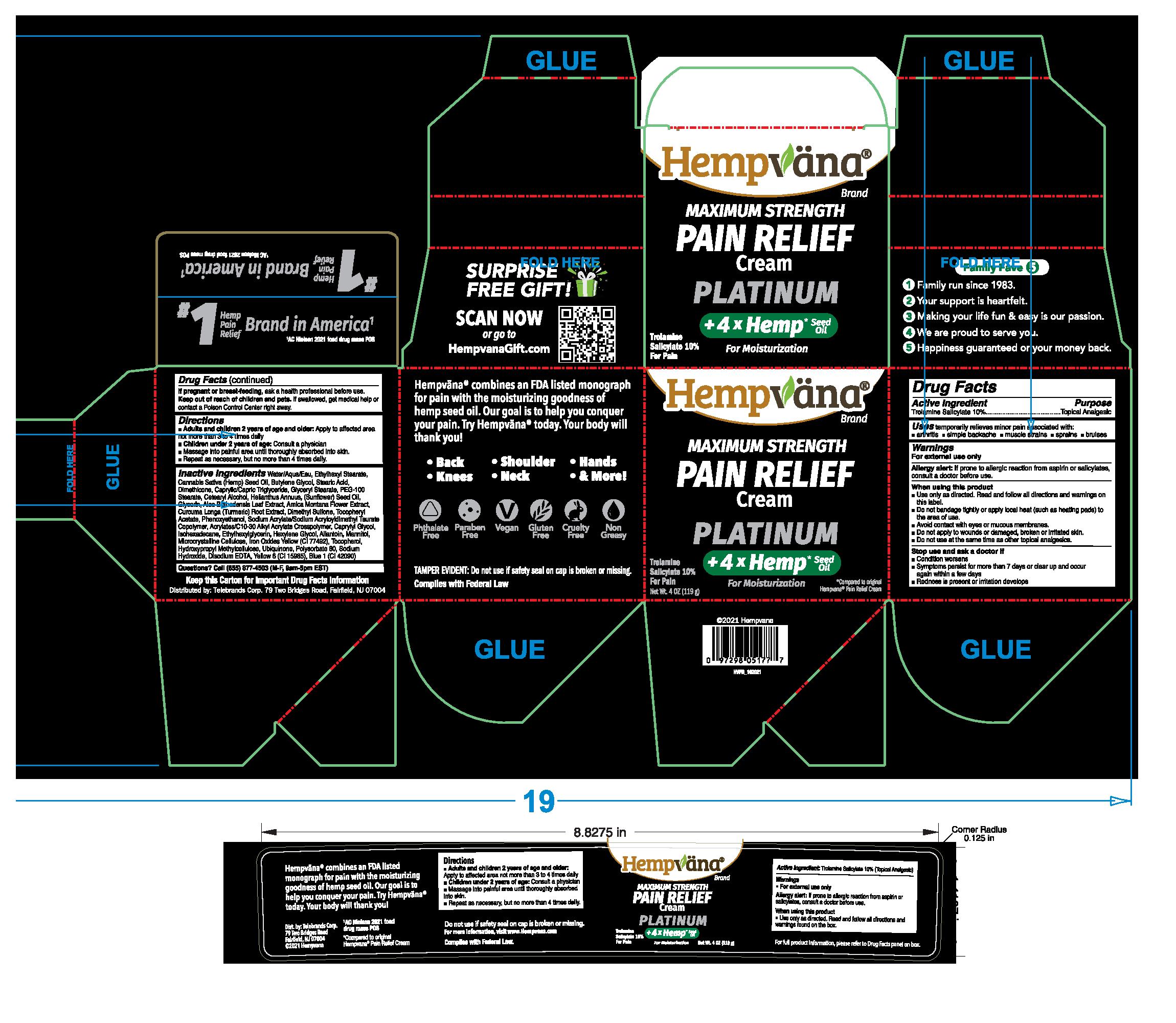
Label: HEMPVANA MAXIMUM STRENGTH PAIN RELIEF - PLATINUM- trolamine salicylate cream
- NDC Code(s): 73287-021-01, 73287-021-02
- Packager: TELEBRANDS CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert:If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin.
- Do not use at the same time as other topical analgesics.
- Directions
-
Inactive ingredients
Water/Aqua/Eau, Ethylhexyl Stearate, Cannabis Sativa (Hemp) Seed Oil, Butylene Glycol, Stearic Acid, DImethicone, Caprylic/Capric Trigylceride, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Helianthus Annuus (Sunflower) Seed Oil, Glycerin, Aloe Barbadensis Leaf Extract, Arnica Montana Flower Extract, Curcuma Longa (Turmeric) Root Extract, Dimethyl Sulfone, Tocopheryl Acetate, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Isohexadecane, Ethylhexylglycerin, Hexylene Glycol, Allantoin, Mannitol, Microcrystalline Cellulose, Iron Oxides Yellow (CI 77492), Tocopherol, Hydroxypropyl Methylcellulose, Ubiquinone, Polysorbate 80, Sodium Hydroxide, Disodium EDTA, Yellow 6 (CI 15985), Blue 1 (CI 42090)
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMPVANA MAXIMUM STRENGTH PAIN RELIEF - PLATINUM
trolamine salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73287-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROLAMINE SALICYLATE (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) TROLAMINE SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) TURMERIC (UNII: 856YO1Z64F) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) UBIDECARENONE (UNII: EJ27X76M46) ALLANTOIN (UNII: 344S277G0Z) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) STEARIC ACID (UNII: 4ELV7Z65AP) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SUNFLOWER OIL (UNII: 3W1JG795YI) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM HYDROXIDE (UNII: 55X04QC32I) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HEXYLENE GLYCOL (UNII: KEH0A3F75J) WATER (UNII: 059QF0KO0R) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) PEG-100 STEARATE (UNII: YD01N1999R) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73287-021-01 1 in 1 CARTON 01/14/2022 1 119 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:73287-021-02 524 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/14/2022 Labeler - TELEBRANDS CORP (177266558) Establishment Name Address ID/FEI Business Operations Neutraderm, Inc. 146224444 manufacture(73287-021)