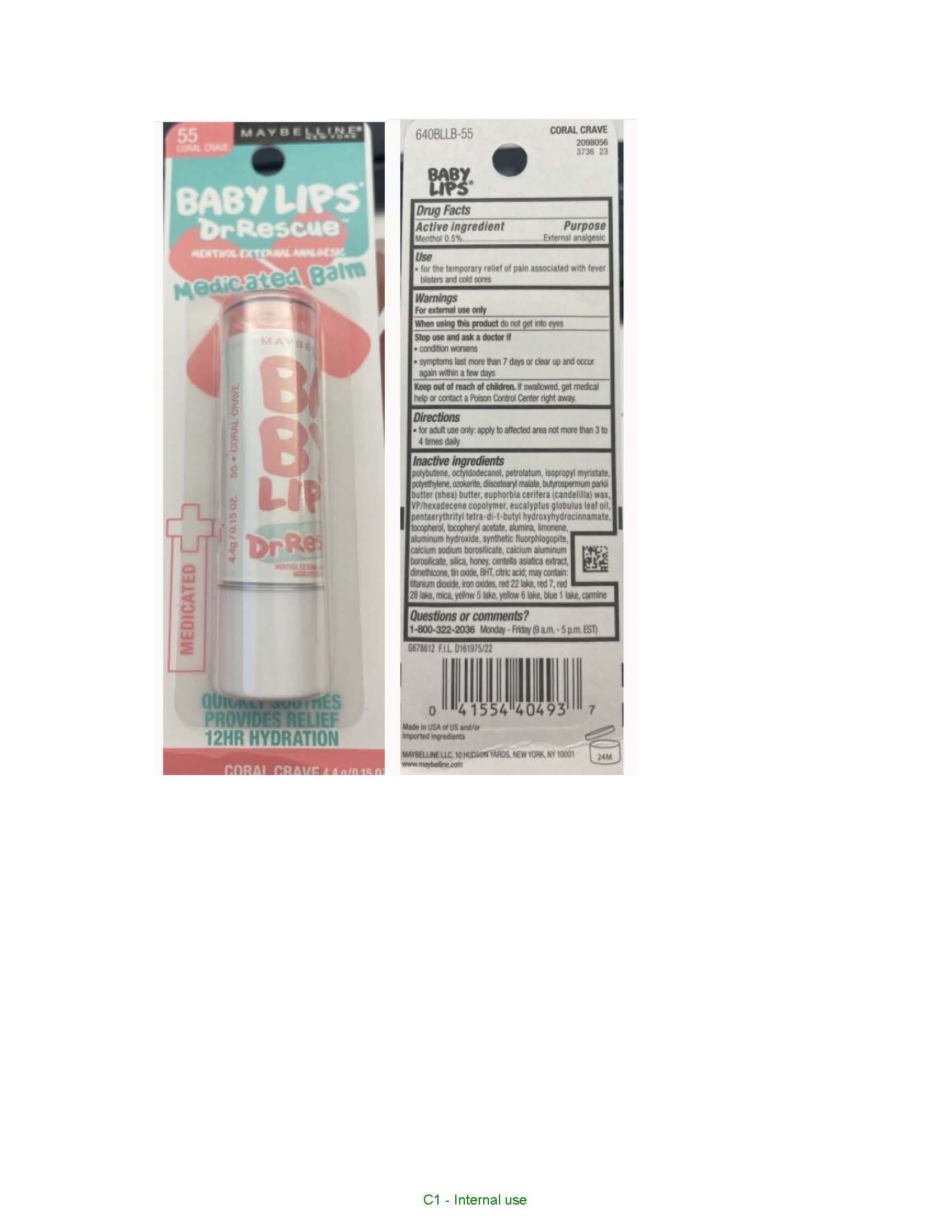
Label: MAYBELLINE NEW YORK BABY LIPS DR RESCURE MENTHOL EXTERNAL ANALGESIC MEDICATED BALM- menthol lipstick
- NDC Code(s): 49967-494-01
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- polybutene, octyldodecanol, petrolatum, isopropyl myristate, polyethylene, ozokerite, diisostearyl malate, butyrospermum parkii (shea) butter, euphorbia cerifera (candelilla) wax, VP/hexadecene copolymer, eucalyptus globulus leaf oil, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, tocopherol, tocopheryl acetate, alumina, limonene, aluminum hydroxide, synthetic fluorphlogopite, calcium sodium borosilicate, calcium aluminum borosilicate, silica, honey, centella asiatica extract, dimethicone, tin oxide, BHT, citric acid, titanium dioxide, iron oxides, red 22 lake, red 7, red 28 lake, mica, yellow 5 lake, yellow 6 lake, blue 1 lake, carmine
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAYBELLINE NEW YORK BABY LIPS DR RESCURE MENTHOL EXTERNAL ANALGESIC MEDICATED BALM
menthol lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-494 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 mg in 1 g Inactive Ingredients Ingredient Name Strength OCTYLDODECANOL (UNII: 461N1O614Y) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) EUCALYPTUS OIL (UNII: 2R04ONI662) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALUMINUM OXIDE (UNII: LMI26O6933) LIMONENE, (+)- (UNII: GFD7C86Q1W) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CALCIUM SODIUM BOROSILICATE (UNII: 4MM76N4WMY) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HONEY (UNII: Y9H1V576FH) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) DIMETHICONE (UNII: 92RU3N3Y1O) STANNIC OXIDE (UNII: KM7N50LOS6) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) MICA (UNII: V8A1AW0880) FD&C YELLOW NO. 6 ALUMINUM LAKE (UNII: GYP6Z2JR6Q) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-494-01 1 in 1 BLISTER PACK 06/01/2016 1 4.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2016 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA PRODUCTS, INC 624244349 manufacture(49967-494)