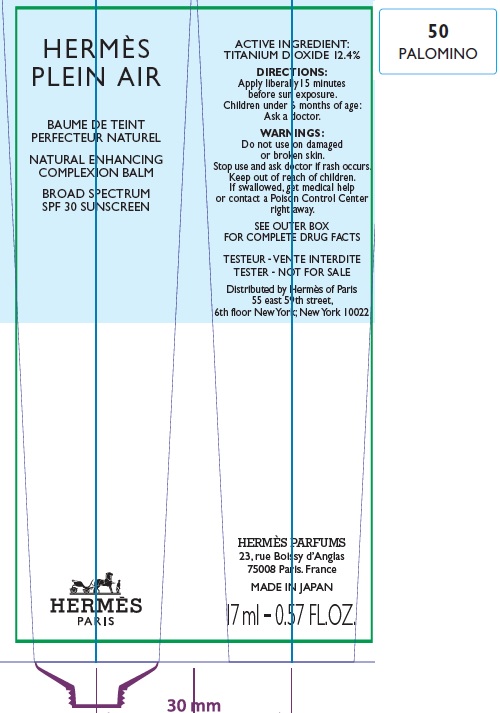
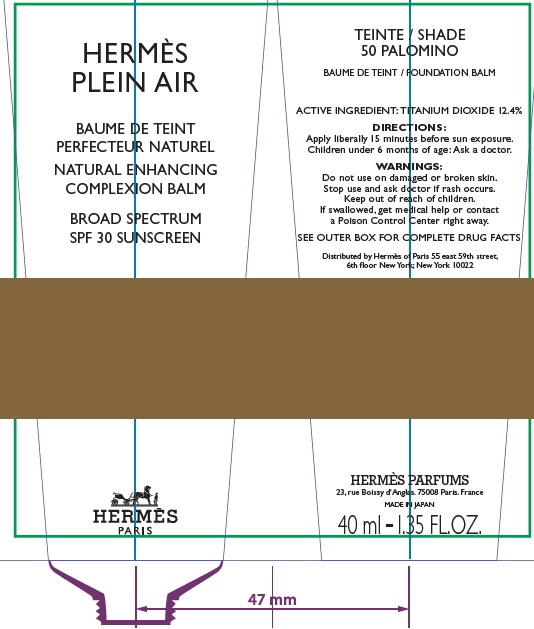
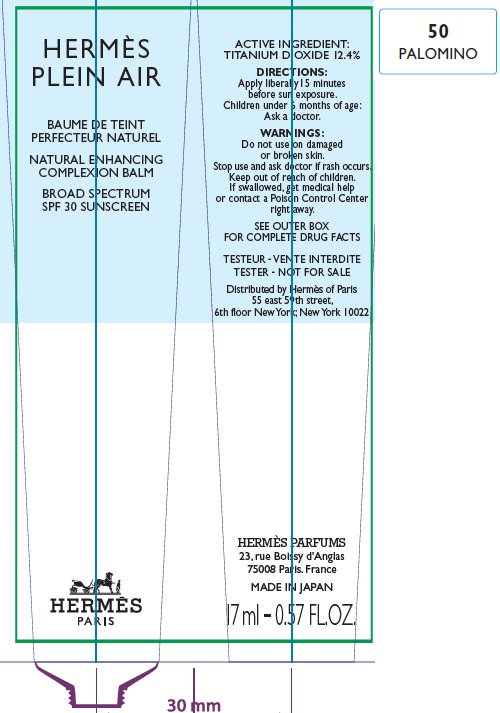
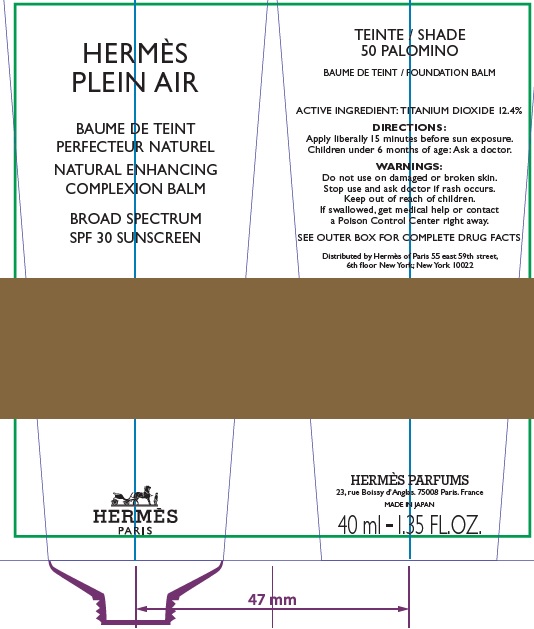
Label: HERMES PLEIN AIR NATURAL ENHANCING COMPLEXION BALM SPF 30 SUNSCREEN 50 PALOMINO- titanium dioxide cream
- NDC Code(s): 82494-005-01, 82494-005-17, 82494-005-40
- Packager: COMPTOIR NOUVEAU DE LA PARFUMERIE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Children under 6 months age: Ask a doctor.
- Use a water-resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Reapply immediately after towel drying.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including : • Limit time in the sun, especially from 10 a.m-2 p.m. • Wear long-sleeved shirts, hats, and sunglasses.
Sun Protection Measures.
-
Inactive ingredients
AQUA (WATER), METHYL TRIMETHICONE, NEOPENTYL GLYCOL DIETHYLHEXANOATE, DIMETHICONE, DIPROPYLENE GLYCOL, PEG-10 DIMETHICONE, CYCLOPENTASILOXANE, VINYL DIMETHICONE/ METHICONE SILSESQUIOXANE CROSSPOLYMER, PENTYLENE GLYCOL, BUTYLENE GLYCOL, GLYCERIN, PHENOXYETHANOL, POLYGLYCERYL-2 DIISOSTEARATE, SORBITOL, PARFUM (FRAGRANCE), DISTEARDIMONIUM HECTORITE, TRIMETHYLSILOXYSILICATE, SODIUM CITRATE, SODIUM CHLORIDE, STEARIC ACID, CAPRYLIC/CAPRIC TRIGLYCERIDE, ALUMINA, TOCOPHEROL, GLYCINE, DIMETHICONE/ VINYL DIMETHICONE CROSSPOLYMER, BENZYL SALICYLATE, SODIUM HYALURONATE, SCUTELLARIA BAICALENSIS ROOT EXTRACT, LIMONENE, OENOTHERA BIENNIS (EVENING PRIMROSE) SEED EXTRACT, MORUS ALBA BARK EXTRACT, GERANIOL, CITRONELLOL, LINALOOL, HYDROXYCITRONELLAL [+/- (MAY CONTAIN) : MICA, CI 77891 (TITANIUM DIOXIDE), CI 77491/CI 77492/ CI 77499 (IRON OXIDES)].
- Other information
- Package Labeling:82494-005-01
- Package Labeling:82494-005-17
- Package Labeling:82494-005-40
-
INGREDIENTS AND APPEARANCE
HERMES PLEIN AIR NATURAL ENHANCING COMPLEXION BALM SPF 30 SUNSCREEN 50 PALOMINO
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82494-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 124 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) DIMETHICONE (UNII: 92RU3N3Y1O) DIPROPYLENE GLYCOL (UNII: E107L85C40) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-2 DIISOSTEARATE (UNII: DG195GP57P) SORBITOL (UNII: 506T60A25R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM OXIDE (UNII: LMI26O6933) TOCOPHEROL (UNII: R0ZB2556P8) GLYCINE (UNII: TE7660XO1C) BENZYL SALICYLATE (UNII: WAO5MNK9TU) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) LIMONENE, (+)- (UNII: GFD7C86Q1W) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) MORUS ALBA BARK (UNII: 7O71A48NDP) GERANIOL (UNII: L837108USY) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82494-005-01 1 mL in 1 BAG; Type 0: Not a Combination Product 01/15/2022 2 NDC:82494-005-17 1 in 1 CARTON 01/15/2022 2 17 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:82494-005-40 1 in 1 CARTON 01/15/2022 3 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/15/2022 Labeler - COMPTOIR NOUVEAU DE LA PARFUMERIE (275137834)