Label: GLUCAGON kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5070-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0002-8031
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 18, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Glucagon for Injection (rDNA origin) is a polypeptide hormone identical to human glucagon that increases blood glucose and relaxes smooth muscle of the gastrointestinal tract. Glucagon is synthesized in a special non-pathogenic laboratory strain of Escherichia coli bacteria that has been genetically altered by the addition of the gene for glucagon.
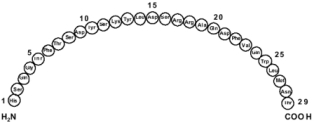
Glucagon is a single-chain polypeptide that contains 29 amino acid residues and has a molecular weight of 3483.
The empirical formula is C153H225N43O49S. The primary sequence of glucagon is shown below.
Crystalline glucagon is a white to off-white powder. It is relatively insoluble in water but is soluble at a pH of less than 3 or more than 9.5.
Glucagon is available for use intravenously, intramuscularly, or subcutaneously in a kit that contains a vial of sterile glucagon and a syringe of sterile diluent. The vial contains 1 mg (1 unit) of glucagon and 49 mg of lactose. Hydrochloric acid may have been added during manufacture to adjust the pH of the glucagon. One International Unit of glucagon is equivalent to 1 mg of glucagon.1 The diluent syringe contains 12 mg/mL of glycerin, Water For Injection, and hydrochloric acid.
-
CLINICAL PHARMACOLOGY
Glucagon increases blood glucose concentration and is used in the treatment of hypoglycemia. Glucagon acts only on liver glycogen, converting it to glucose.
Glucagon administered through a parenteral route relaxes smooth muscle of the stomach, duodenum, small bowel, and colon.
Pharmacokinetics
Glucagon has been studied following intramuscular, subcutaneous, and intravenous administration in adult volunteers. Administration of the intravenous glucagon showed dose proportionality of the pharmacokinetics between 0.25 and 2.0 mg. Calculations from a 1 mg dose showed a small volume of distribution (mean, 0.25 L/kg) and a moderate clearance (mean, 13.5 mL/min/kg). The half-life was short, ranging from 8 to 18 minutes.
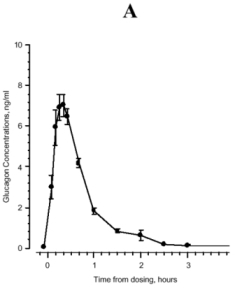
Maximum plasma concentrations of 7.9 ng/mL were achieved approximately 20 minutes after subcutaneous administration (see Figure 1A). With intramuscular dosing, maximum plasma concentrations of 6.9 ng/mL were attained approximately 13 minutes after dosing.
Glucagon is extensively degraded in liver, kidney, and plasma. Urinary excretion of intact glucagon has not been measured.
Pharmacodynamics
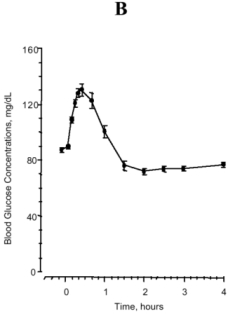
In a study of 25 volunteers, a subcutaneous dose of 1 mg glucagon resulted in a mean peak glucose concentration of 136 mg/dL 30 minutes after injection (see Figure 1B). Similarly, following intramuscular injection, the mean peak glucose level was 138 mg/dL, which occurred at 26 minutes after injection. No difference in maximum blood glucose concentration between animal-sourced and rDNA glucagon was observed after subcutaneous and intramuscular injection.
-
INDICATIONS AND USAGE
For the treatment of hypoglycemia:
Glucagon is indicated as a treatment for severe hypoglycemia.
Because patients with type 1 diabetes may have less of an increase in blood glucose levels compared with a stable type 2 patient, supplementary carbohydrate should be given as soon as possible, especially to a pediatric patient.
For use as a diagnostic aid:
Glucagon is indicated as a diagnostic aid in the radiologic examination of the stomach, duodenum, small bowel, and colon when diminished intestinal motility would be advantageous.
Glucagon is as effective for this examination as are the anticholinergic drugs. However, the addition of the anticholinergic agent may result in increased side effects.
- CONTRAINDICATIONS
-
WARNINGS
Glucagon should be administered cautiously to patients with a history suggestive of insulinoma, pheochromocytoma, or both. In patients with insulinoma, intravenous administration of glucagon may produce an initial increase in blood glucose; however, because of glucagon's hyperglycemic effect the insulinoma may release insulin and cause subsequent hypoglycemia. A patient developing symptoms of hypoglycemia after a dose of glucagon should be given glucose orally, intravenously, or by gavage, whichever is most appropriate.
Exogenous glucagon also stimulates the release of catecholamines. In the presence of pheochromocytoma, glucagon can cause the tumor to release catecholamines, which may result in a sudden and marked increase in blood pressure. If a patient develops a sudden increase in blood pressure, 5 to 10 mg of phentolamine mesylate may be administered intravenously in an attempt to control the blood pressure.
-
PRECAUTIONS
Information for Patients
Refer patients and family members to the attached Information for the User for instructions describing the method of preparing and injecting glucagon. Advise the patient and family members to become familiar with the technique of preparing glucagon before an emergency arises. Instruct patients to use 1 mg (1 unit) for adults and 1/2 the adult dose (0.5 mg) [0.5 unit] for pediatric patients weighing less than 44 lb (20 kg).
Patients and family members should be informed of the following measures to prevent hypoglycemic reactions due to insulin:
- Reasonable uniformity from day to day with regard to diet, insulin, and exercise.
- Careful adjustment of the insulin program so that the type (or types) of insulin, dose, and time (or times) of administration are suited to the individual patient.
- Frequent testing of the blood or urine for glucose so that a change in insulin requirements can be foreseen.
- Routine carrying of sugar, candy, or other readily absorbable carbohydrate by the patient so that it may be taken at the first warning of an oncoming reaction.
To prevent severe hypoglycemia, patients and family members should be informed of the symptoms of mild hypoglycemia and how to treat it appropriately.
Family members should be informed to arouse the patient as quickly as possible because prolonged hypoglycemia may result in damage to the central nervous system. Glucagon or intravenous glucose should awaken the patient sufficiently so that oral carbohydrates may be taken.
Patients should be advised to inform their physician when hypoglycemic reactions occur so that the treatment regimen may be adjusted if necessary.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Because glucagon is usually given in a single dose and has a very short half-life, no studies have been done regarding carcinogenesis. In a series of studies examining effects on the bacterial mutagenesis (Ames) assay, it was determined that an increase in colony counts was related to technical difficulties in running this assay with peptides and was not due to mutagenic activities of the glucagon.
Pregnancy
Pregnancy Category B — Reproduction studies have not been performed with recombinant glucagon. However, studies with animal-sourced glucagon were performed in rats at doses up to 2 mg/kg glucagon administered two times a day (up to 40 times the human dose based on body surface area, mg/m2), and have revealed no evidence of impaired fertility or harm to the fetus due to glucagon. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when glucagon is administered to a nursing woman. If the drug is excreted in human milk during its short half-life, it will be hydrolyzed and absorbed like any other polypeptide. Glucagon is not active when taken orally because it is destroyed in the gastrointestinal tract before it can be absorbed.
Pediatric Use
Geriatric Use
Clinical studies of glucagon did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Severe adverse reactions are very rare, although nausea and vomiting may occur occasionally. These reactions may also occur with hypoglycemia. Generalized allergic reactions have been reported (see WARNINGS). In a three month controlled study of 75 volunteers comparing animal-sourced glucagon with glucagon manufactured through rDNA technology, no glucagon-specific antibodies were detected in either treatment group.
-
OVERDOSAGE
Signs and Symptoms — If overdosage occurs, nausea, vomiting, gastric hypotonicity, and diarrhea would be expected without causing consequential toxicity.
Intravenous administration of glucagon has been shown to have positive inotropic and chronotropic effects. A transient increase in both blood pressure and pulse rate may occur following the administration of glucagon. Patients taking β-blockers might be expected to have a greater increase in both pulse and blood pressure, an increase of which will be transient because of glucagon's short half-life. The increase in blood pressure and pulse rate may require therapy in patients with pheochromocytoma or coronary artery disease.
When glucagon was given in large doses to patients with cardiac disease, investigators reported a positive inotropic effect. These investigators administered glucagon in doses of 0.5 to 16 mg/hour by continuous infusion for periods of 5 to 166 hours. Total doses ranged from 25 to 996 mg, and a 21-month-old infant received approximately 8.25 mg in 165 hours. Side effects included nausea, vomiting, and decreasing serum potassium concentration. Serum potassium concentration could be maintained within normal limits with supplemental potassium.
The intravenous median lethal dose for glucagon in mice and rats is approximately 300 mg/kg and 38.6 mg/kg, respectively.
Because glucagon is a polypeptide, it would be rapidly destroyed in the gastrointestinal tract if it were to be accidentally ingested.
Treatment — To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
In view of the extremely short half-life of glucagon and its prompt destruction and excretion, the treatment of overdosage is symptomatic, primarily for nausea, vomiting, and possible hypokalemia.
If the patient develops a dramatic increase in blood pressure, 5 to 10 mg of phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of glucagon; it is extremely unlikely that one of these procedures would ever be indicated.
-
DOSAGE AND ADMINISTRATION
General Instructions for Use:
- The diluent is provided for use only in the preparation of glucagon for parenteral injection and for no other use.
- Glucagon should not be used at concentrations greater than 1 mg/mL (1 unit/mL).
- Reconstituted glucagon should be used immediately. Discard any unused portion.
- Reconstituted glucagon solutions should be used only if they are clear and of a water-like consistency.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Directions for Treatment of Severe Hypoglycemia:
Severe hypoglycemia should be treated initially with intravenous glucose, if possible.
- If parenteral glucose can not be used, dissolve the lyophilized glucagon using the accompanying diluting solution and use immediately.
- For adults and for pediatric patients weighing more than 44 lb (20 kg), give 1 mg (1 unit) by subcutaneous, intramuscular, or intravenous injection.
- For pediatric patients weighing less than 44 lb (20 kg), give 0.5 mg (0.5 unit) or a dose equivalent to 20 to 30 μg/kg.2-6
- Discard any unused portion.
- An unconscious patient will usually awaken within 15 minutes following the glucagon injection. If the response is delayed, there is no contraindication to the administration of an additional dose of glucagon; however, in view of the deleterious effects of cerebral hypoglycemia emergency aid should be sought so that parenteral glucose can be given.
- After the patient responds, supplemental carbohydrate should be given to restore liver glycogen and to prevent secondary hypoglycemia.
Directions for Use as a Diagnostic Aid:
Dissolve the lyophilized glucagon using the accompanying diluting solution and use immediately. Discard any unused portion.
The doses in the following table may be administered for relaxation of the stomach, duodenum, and small bowel, depending on the onset and duration of effect required for the examination. Since the stomach is less sensitive to the effect of glucagon, 0.5 mg (0.5 units) IV or 2 mg (2 units) IM are recommended.
Dose Route of Administration Time of Onset of Action Approximate Duration of Effect * Administration of 2 mg (2 units) doses produces a higher incidence of nausea and vomiting than do lower doses.
0.25-0.5 mg (0.25-0.5 units) IV 1 minute 9-17 minutes 1-mg (1 unit) IM 8-10 minutes 12-27 minutes 2 mg* (2 units) IV 1 minute 22-25 minutes 2 mg* (2 units) IM 4-7 minutes 21-32 minutes For examination of the colon, it is recommended that a 2 mg (2 units) dose be administered intramuscularly approximately 10 minutes prior to the procedure. Colon relaxation and reduction of patient discomfort may allow the radiologist to perform a more satisfactory examination.
-
HOW SUPPLIED
Glucagon Emergency Kit for Low Blood Sugar (Glucagon for Injection [rDNA origin]) (MS8031):
1 mg (1 unit) — (VL7529), with 1 mL of diluting solution (Hyporet®* HY7530) (1s) NDC 54868-5070-0
*Hyporet® (disposable syringe, Lilly).
Stability and Storage:
Before Reconstitution — Vials of Glucagon, as well as the Diluting Solution for Glucagon, may be stored at controlled room temperature 20° to 25°C (68° to 77°F)[see USP].
The USP defines controlled room temperature by the following: A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15° and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses.
-
REFERENCES
- Drug Information for the Health Care Professional. 18th ed. Rockville, Maryland: The United States Pharmacopeial Convention, Inc; 1998; I:1512.
- Gibbs et al: Use of glucagon to terminate insulin reactions in diabetic children. Nebr Med J 1958;43:56-57.
- Cornblath M, et al: Studies of carbohydrate metabolism in the newborn: Effect of glucagon on concentration of sugar in capillary blood of newborn infant. Pediatrics 1958;21:885-892.
- Carson MJ, Koch R: Clinical studies with glucagon in children. J Pediatr 1955;47:161-170.
- Shipp JC, et al: Treatment of insulin hypoglycemia in diabetic campers. Diabetes 1964;13:645-648.
- Aman J, Wranne L: Hypoglycemia in childhood diabetes II: Effect of subcutaneous or intramuscular injection of different doses of glucagon. Acta Pediatr Scand 1988;77:548-553.
- SPL UNCLASSIFIED SECTION
-
INFORMATION FOR THE USER
GLUCAGON
FOR INJECTION
(rDNA ORIGIN)BECOME FAMILIAR WITH THE FOLLOWING INSTRUCTIONS BEFORE AN EMERGENCY ARISES. DO NOT USE THIS KIT AFTER DATE STAMPED ON THE BOTTLE LABEL. IF YOU HAVE QUESTIONS CONCERNING THE USE OF THIS PRODUCT, CONSULT A DOCTOR, NURSE OR PHARMACIST.
Make sure that your relatives or close friends know that if you become unconscious, medical assistance must always be sought. Glucagon may have been prescribed so that members of your household can give the injection if you become hypoglycemic and are unable to take sugar by mouth. If you are unconscious, glucagon can be given while awaiting medical assistance.
Show your family members and others where you keep this kit and how to use it. They need to know how to use it before you need it. They can practice giving a shot by giving you your normal insulin shots. It is important that they practice. A person who has never given a shot probably will not be able to do it in an emergency.
IMPORTANT
- Act quickly. Prolonged unconsciousness may be harmful.
- These simple instructions will help you give glucagon successfully.
- Turn patient on his/her side to prevent patient from choking.
- The contents of the syringe are inactive. You must mix the contents of the syringe with the glucagon in the accompanying bottle before giving injection. (See DIRECTIONS FOR USE below.)
- Do not prepare Glucagon for Injection until you are ready to use it.
WARNING: THE PATIENT MAY BE IN A COMA FROM SEVERE HYPERGLYCEMIA (HIGH BLOOD GLUCOSE) RATHER THAN HYPOGLYCEMIA. IN SUCH A CASE, THE PATIENT WILL NOT RESPOND TO GLUCAGON AND REQUIRES IMMEDIATE MEDICAL ATTENTION.
INDICATIONS FOR USE
Use glucagon to treat insulin coma or insulin reaction resulting from severe hypoglycemia (low blood sugar). Symptoms of severe hypoglycemia include disorientation, unconsciousness, and seizures or convulsions. Give glucagon if (1) the patient is unconscious (2) the patient is unable to eat sugar or a sugar-sweetened product (3) the patient is having a seizure, or (4) repeated administration of sugar or a sugar-sweetened product such as a regular soft drink or fruit juice does not improve the patient's condition. Milder cases of hypoglycemia should be treated promptly by eating sugar or a sugar-sweetened product. (See INFORMATION ON HYPOGLYCEMIA below for more information on the symptoms of hypoglycemia.) Glucagon is not active when taken orally.
DIRECTIONS FOR USE
TO PREPARE GLUCAGON FOR INJECTION
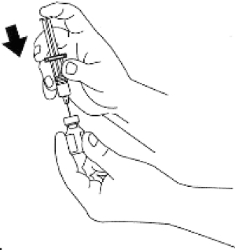
- Remove the flip-off seal from the bottle of glucagon. Wipe rubber stopper
on bottle with alcohol swab.
- Remove the needle protector from the syringe, and inject the entire
contents of the syringe into the bottle of glucagon. DO NOT REMOVE THE PLASTIC
CLIP FROM THE SYRINGE. Remove syringe from the bottle.
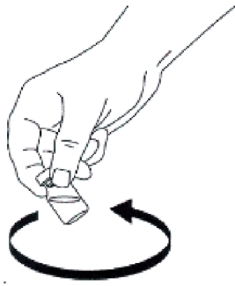
- Swirl bottle gently until glucagon dissolves completely. GLUCAGON SHOULD
NOT BE USED UNLESS THE SOLUTION IS CLEAR AND OF A WATER-LIKE CONSISTENCY.
TO INJECT GLUCAGON
Use Same Technique as for Injecting Insulin
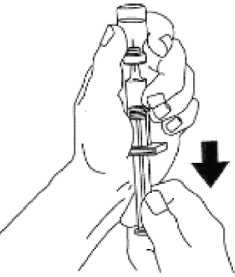
- Using the same syringe, hold bottle upside down and, making sure the
needle tip remains in solution, gently withdraw all of the solution (1 mg
mark on syringe) from bottle. The plastic clip on the syringe will prevent
the rubber stopper from being pulled out of the syringe; however, if the plastic
plunger rod separates from the rubber stopper, simply reinsert the rod by
turning it clockwise. The usual adult dose is 1 mg (1 unit). For children
weighing less than 44 lb (20 kg), give 1/2 adult dose (0.5 mg). For children,
withdraw 1/2 of the solution from the bottle (0.5 mg mark on syringe). DISCARD
UNUSED PORTION.
USING THE FOLLOWING DIRECTIONS, INJECT GLUCAGON IMMEDIATELY AFTER MIXING.
- Cleanse injection site on buttock, arm, or thigh with alcohol swab.
- Insert the needle into the loose tissue under the cleansed injection site, and inject all (or 1/2 for children weighing less than 44 lb) of the glucagon solution. THERE IS NO DANGER OF OVERDOSE. Apply light pressure at the injection site, and withdraw the needle. Press an alcohol swab against the injection site.
- Turn the patient on his/her side. When an unconscious person awakens, he/she may vomit. Turning the patient on his/her side will prevent him/her from choking.
- FEED THE PATIENT AS SOON AS HE/SHE AWAKENS AND IS ABLE TO SWALLOW. Give the patient a fast-acting source of sugar (such as a regular soft drink or fruit juice) and a long-acting source of sugar (such as crackers and cheese or a meat sandwich). If the patient does not awaken within 15 minutes, give another dose of glucagon and INFORM A DOCTOR OR EMERGENCY SERVICES IMMEDIATELY.
- Even if the glucagon revives the patient, his/her doctor should be promptly notified. A doctor should be notified whenever severe hypoglycemic reactions occur.
INFORMATION ON HYPOGLYCEMIA
Early symptoms of hypoglycemia (low blood glucose) include:
- sweating
- drowsiness
- dizziness
- sleep disturbances
- palpitation
- anxiety
- tremor
- blurred vision
- hunger
- slurred speech
- restlessness
- depressed mood
- tingling in the hands, feet, lips, or tongue
- irritability
- lightheadedness
- abnormal behavior
- inability to concentrate
- unsteady movement
- headache
- personality changes
If not treated, the patient may progress to severe hypoglycemia that can include:
- disorientation
- seizures
- unconsciousness
- death
The occurrence of early symptoms calls for prompt and, if necessary, repeated administration of some form of carbohydrate. Patients should always carry a quick source of sugar, such as candy mints or glucose tablets. The prompt treatment of mild hypoglycemic symptoms can prevent severe hypoglycemic reactions. If the patient does not improve or if administration of carbohydrate is impossible, glucagon should be given or the patient should be treated with intravenous glucose at a medical facility. Glucagon, a naturally occurring substance produced by the pancreas, is helpful because it enables the patient to produce his/her own blood glucose to correct the hypoglycemia.
POSSIBLE PROBLEMS WITH GLUCAGON TREATMENT
Severe side effects are very rare, although nausea and vomiting may occur occasionally.
A few people may be allergic to glucagon or to one of the inactive ingredients in glucagon, or may experience rapid heart beat for a short while.
If you experience any other reactions which are likely to have been caused by glucagon, please contact your doctor.
STORAGE
Before dissolving glucagon with diluting solution — Store the kit at controlled room temperature between 20° to 25°C (68° to 77°F).
After dissolving glucagon with diluting solution — Should be used immediately. Discard any unused portion. Solutions should be clear and of a water-like consistency at time of use.
Literature revised February 18, 2005
Eli Lilly and Company
Indianapolis, IN 46285, USA
PA 2285 AMP
Copyright © 1999, 2005, Eli Lilly and Company. All rights reserved.
- PACKAGE LABEL – Glucagon 1 mg Emergency Kit 1ct
-
INGREDIENTS AND APPEARANCE
GLUCAGON
glucagon kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5070(NDC:0002-8031) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5070-0 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 mL Part 2 1 SYRINGE 1 mL Part 1 of 2 GLUCAGON
glucagon injection, powder, for solutionProduct Information Route of Administration INTRAVENOUS, INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength glucagon (UNII: 76LA80IG2G) (glucagon - UNII:76LA80IG2G) glucagon 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength lactose (UNII: J2B2A4N98G) 49 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020928 05/24/2004 Part 2 of 2 DILUTING SOLUTION FOR GLUCAGON
diluent injection, solutionProduct Information Route of Administration INTRAVENOUS, INTRAMUSCULAR, SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength glycerin (UNII: PDC6A3C0OX) 12 mg in 1 mL water (UNII: 059QF0KO0R) hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020928 05/24/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020928 05/24/2004 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel