Label: PAULAS CHOICE RESIST SKIN RESTORING MOISTURIZER SPF 50- avobenzone, homosalate,octinoxate, octisalate, oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 76144-797-00 - Packager: Paula’s Choice, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INSTRUCTIONS FOR USE
Apply liberally 15 minutes befor esun exposure.
Use a water-resistant sunscreen if swimming or ssweating:- immediately after towel drying. At least every two hours.
Children under 6 months of age: Ask a doctor.
Sun Protecction Measures. Speding time in the sun increases your risk of skin cancer and early skin aging, To decreas this risk, regularly use a sunsscreen with a broad spectrum SPF 15 or higher and other sun protection measures, including: -Limit time in the sun, especially from 10AM-2PM. -Wear long-sleeve shirts, pants, hats and sunglasses.
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water (Aqua), Silica, Glycerin, Cetearyl Alcohol, Dimethicone, Butylene Glycol, Pentylene Glycol, Potassium Cetyl Phosphate, Cyclopentasiloxane, Pyrus Malus (Apple) Fruit Extract, VP/Eicosene Copolymer, Butyrospermum Parkii (Shea) Butter, Allantoin, Niacinamide, Tocopherol, Glycyrrhiza Glabra (Licorice) Root Extract, Aloe Barbadensis Leaf Juice, Atractylodes Macrocephala Root Powder, Avena Sativa (Oat) Kernel Extract, Coffea Arabica (Coffee) Seed Extract, Portulaca Oleracea Extract, Mahonia Aquifolium Root Extract, Diethylhexyl Syringylidenemalonate, Sarcosine, Capryloyl Glycine, Maltooligosyl Glucoside, Cetearyl Glucoside, Dimethiconol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Hydrogenated Starch Hydrolysate, Hexylene Glycol, 4-T-Butylcyclohexanol, Glyceryl Stearate, PEG-100 Stearate, Sodium Hydroxide, Xanthan Gum, Disodium EDTA, Phenoxyethanol, Caprylyl Glycol, Ethylhexylglycerin.
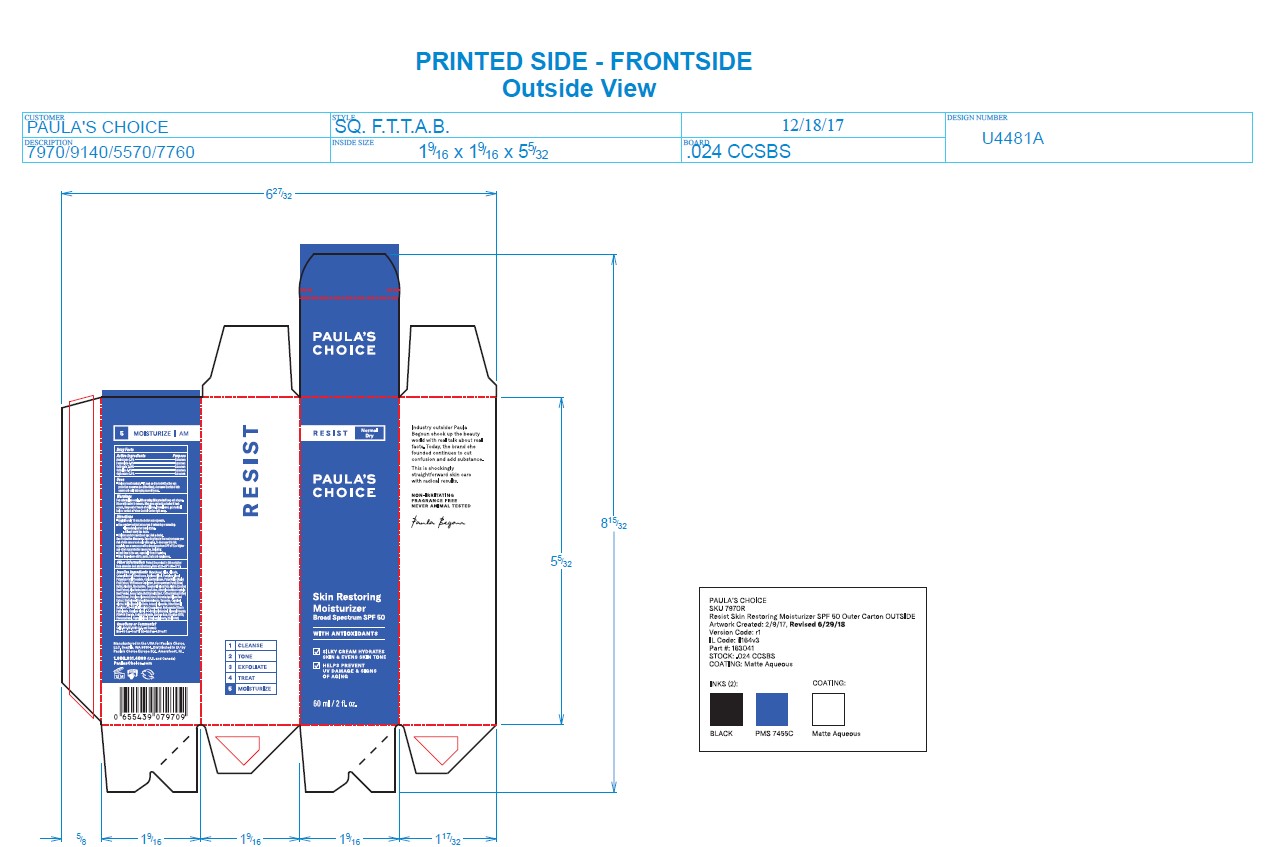
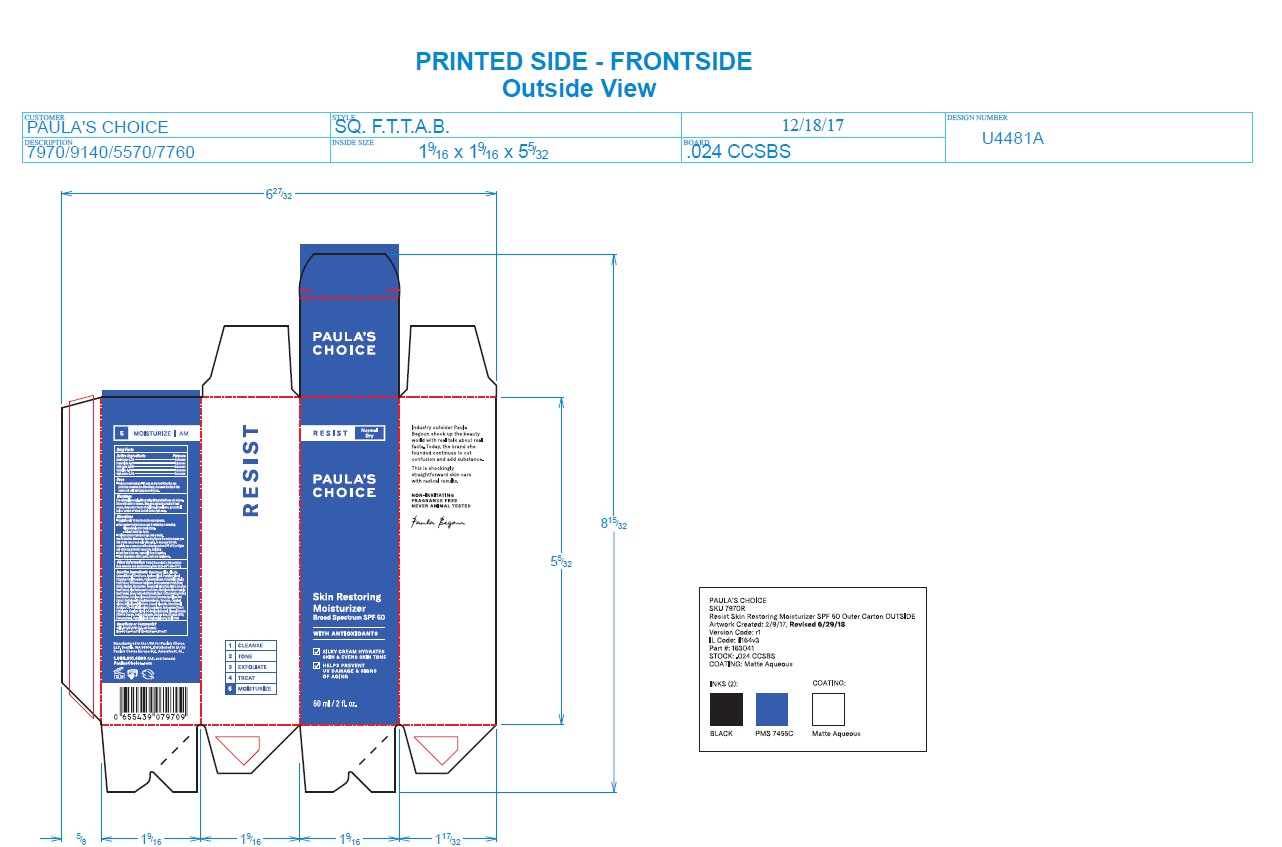
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAULAS CHOICE RESIST SKIN RESTORING MOISTURIZER SPF 50
avobenzone, homosalate,octinoxate, octisalate, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76144-797 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 74.9 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SARCOSINE (UNII: Z711V88R5F) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) HYDROGENATED STARCH HYDROLYSATE (UNII: 27F77DSJ5V) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) EDETATE DISODIUM (UNII: 7FLD91C86K) PORTULACA OLERACEA SEED (UNII: M08917G08Z) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) MALTOOLIGOSYL GLUCOSIDE (UNII: N91S91EFOG) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) DIMETHICONE (UNII: 92RU3N3Y1O) ALOE VERA LEAF (UNII: ZY81Z83H0X) ATRACTYLODES MACROCEPHALA ROOT (UNII: 08T3N29QJB) MAHONIA AQUIFOLIUM ROOT (UNII: 746TB9VNDP) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) HEXYLENE GLYCOL (UNII: KEH0A3F75J) GLYCERYL STEARATE/PEG-75 STEARATE (UNII: 6PSS3TQ3UY) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE (UNII: NMQ347994Z) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PENTYLENE GLYCOL (UNII: 50C1307PZG) ALLANTOIN (UNII: 344S277G0Z) NIACINAMIDE (UNII: 25X51I8RD4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) AVENA SATIVA LEAF (UNII: 206PI19V7R) COFFEA ARABICA SEED, ROASTED (UNII: 9H58JRT35E) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) MALUS DOMESTICA FLOWER (UNII: EF626V855K) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76144-797-00 60 mL in 1 CARTON; Type 0: Not a Combination Product 07/10/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/10/2017 Labeler - Paula’s Choice, LLC. (029583981)