Label: DEEP ACNE- sulfur gel
- NDC Code(s): 68479-240-00, 68479-240-02
- Packager: Dermalogica, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Use
- Warnings
- Directions
-
Inactive ingredients
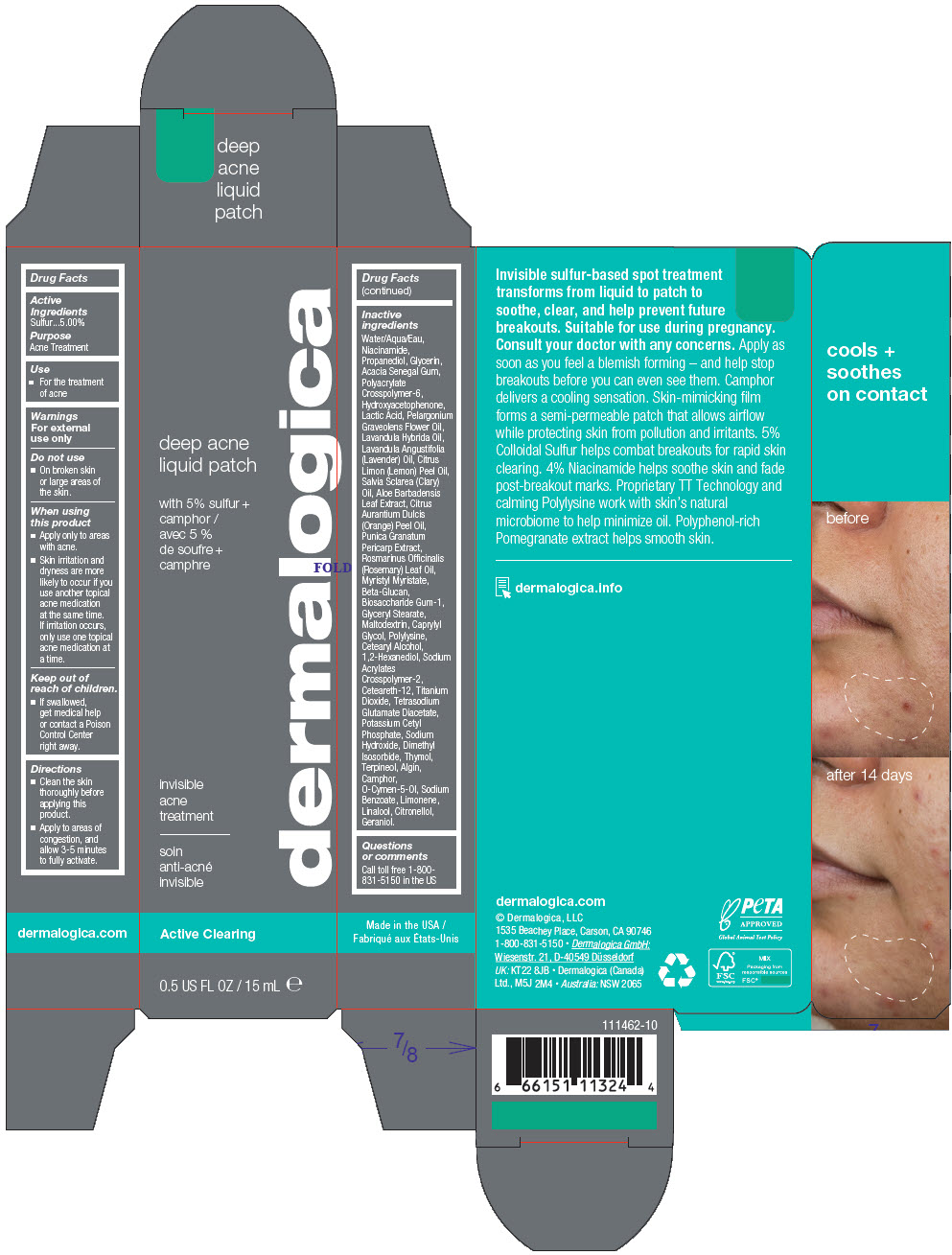
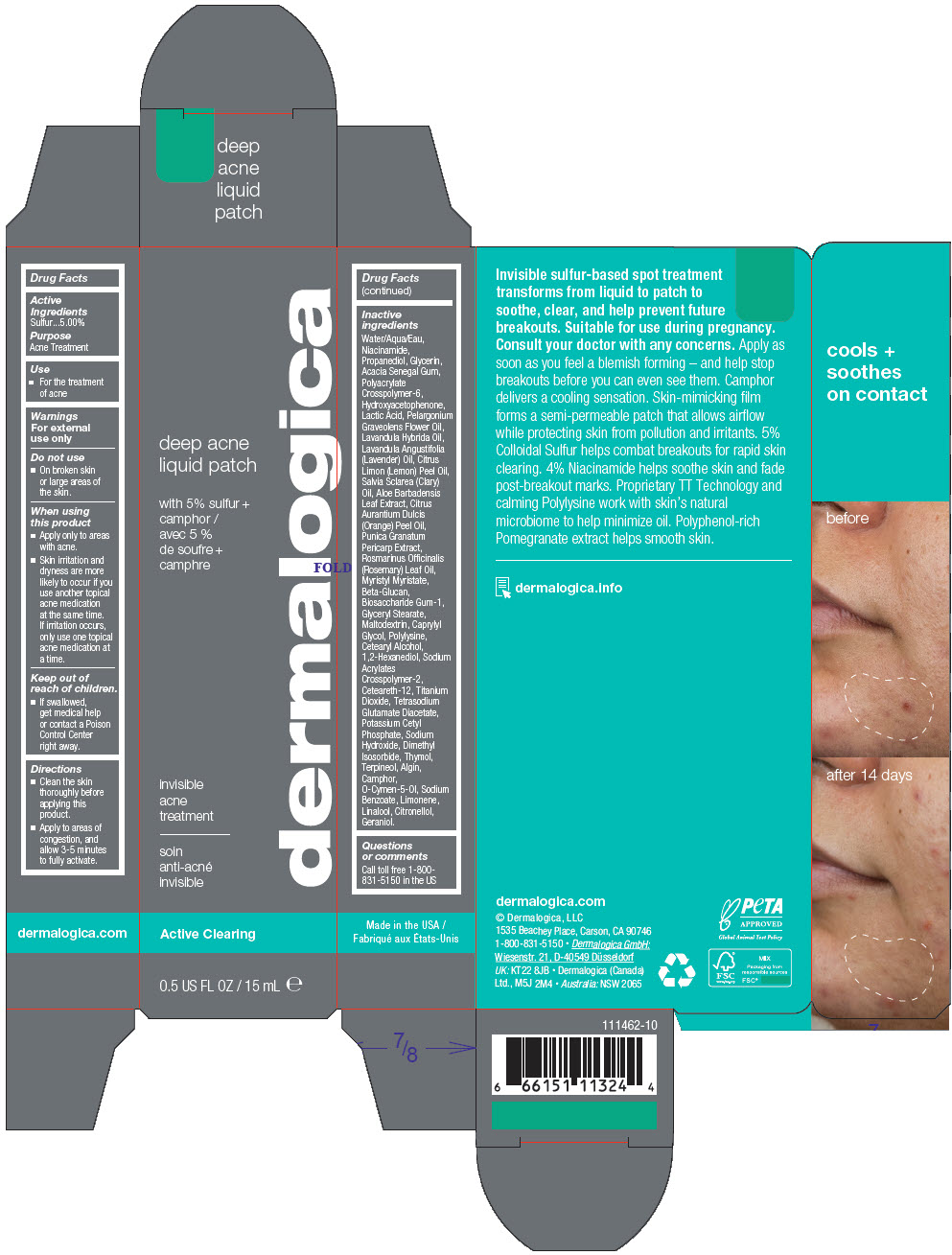
Water/Aqua/Eau, Niacinamide, Propanediol, Glycerin, Acacia Senegal Gum, Polyacrylate Crosspolymer-6, Hydroxyacetophenone, Lactic Acid, Pelargonium Graveolens Flower Oil, Lavandula Hybrida Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Limon (Lemon) Peel Oil, Salvia Sclarea (Clary) Oil, Aloe Barbadensis Leaf Extract, Citrus Aurantium Dulcis (Orange) Peel Oil, Punica Granatum Pericarp Extract, Rosmarinus Officinalis (Rosemary) Leaf Oil, Myristyl Myristate, Beta-Glucan, Biosaccharide Gum-1, Glyceryl Stearate, Maltodextrin, Caprylyl Glycol, Polylysine, Cetearyl Alcohol, 1,2-Hexanediol, Sodium Acrylates Crosspolymer-2, Ceteareth-12, Titanium Dioxide, Tetrasodium Glutamate Diacetate, Potassium Cetyl Phosphate, Sodium Hydroxide, Dimethyl Isosorbide, Thymol, Terpineol, Algin, Camphor, O-Cymen-5-Ol, Sodium Benzoate, Limonene, Linalool, Citronellol, Geraniol.
- Questions or comments
- PRINCIPAL DISPLAY PANEL - 15 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
DEEP ACNE
sulfur gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-240 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 5 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Niacinamide (UNII: 25X51I8RD4) Propanediol (UNII: 5965N8W85T) Glycerin (UNII: PDC6A3C0OX) ACACIA (UNII: 5C5403N26O) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) Myristyl Myristate (UNII: 4042ZC00DY) Maltodextrin (UNII: 7CVR7L4A2D) Dimethyl Isosorbide (UNII: SA6A6V432S) Sodium Acrylates Crosspolymer-2 (UNII: D3HPR4WW6F) Hydroxyacetophenone (UNII: G1L3HT4CMH) Ceteareth-12 (UNII: 7V4MR24V5P) LACTIC ACID, DL- (UNII: 3B8D35Y7S4) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALOE VERA LEAF (UNII: ZY81Z83H0X) o-Cymen-5-ol (UNII: H41B6Q1I9L) Titanium Dioxide (UNII: 15FIX9V2JP) Tetrasodium Glutamate Diacetate (UNII: 5EHL50I4MY) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) Pelargonium Graveolens Flower Oil (UNII: 3K0J1S7QGC) SODIUM ALGINATE (UNII: C269C4G2ZQ) Terpineol (UNII: R53Q4ZWC99) Thymol (UNII: 3J50XA376E) POMEGRANATE FRUIT RIND (UNII: RS999V57DU) LAVENDER OIL (UNII: ZBP1YXW0H8) LAVANDIN OIL (UNII: 9RES347CKG) Sodium Benzoate (UNII: OJ245FE5EU) 1,2-Hexanediol (UNII: TR046Y3K1G) Biosaccharide Gum-1 (UNII: BB4PU4V09H) Caprylyl Glycol (UNII: 00YIU5438U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) CAMPHOR (NATURAL) (UNII: N20HL7Q941) LEMON OIL, DISTILLED (UNII: ET5GD00TRP) ROSEMARY OIL (UNII: 8LGU7VM393) CLARY SAGE OIL (UNII: 87L0D4U3M0) Sodium Hydroxide (UNII: 55X04QC32I) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) Geraniol (UNII: L837108USY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-240-02 1 in 1 CARTON 12/01/2022 1 15 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68479-240-00 2 mL in 1 POUCH; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 12/01/2022 Labeler - Dermalogica, LLC. (177698560) Establishment Name Address ID/FEI Business Operations McKenna 090631412 MANUFACTURE(68479-240)