Label: TOUCHPOINT BIODEGRADABLE SANITIZING WIPES- benzalkonium chloride cloth
-
NDC Code(s):
70924-004-01,
70924-004-02,
70924-004-03,
70924-004-04, view more70924-004-05, 70924-004-06
- Packager: Innocore Sales & Marketing Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
Touch
Point TMBiodegradable
Sanitizing Wipes
840 Wipes
Fragrance Free
Kills 99.99% of most common germs that may cause illness
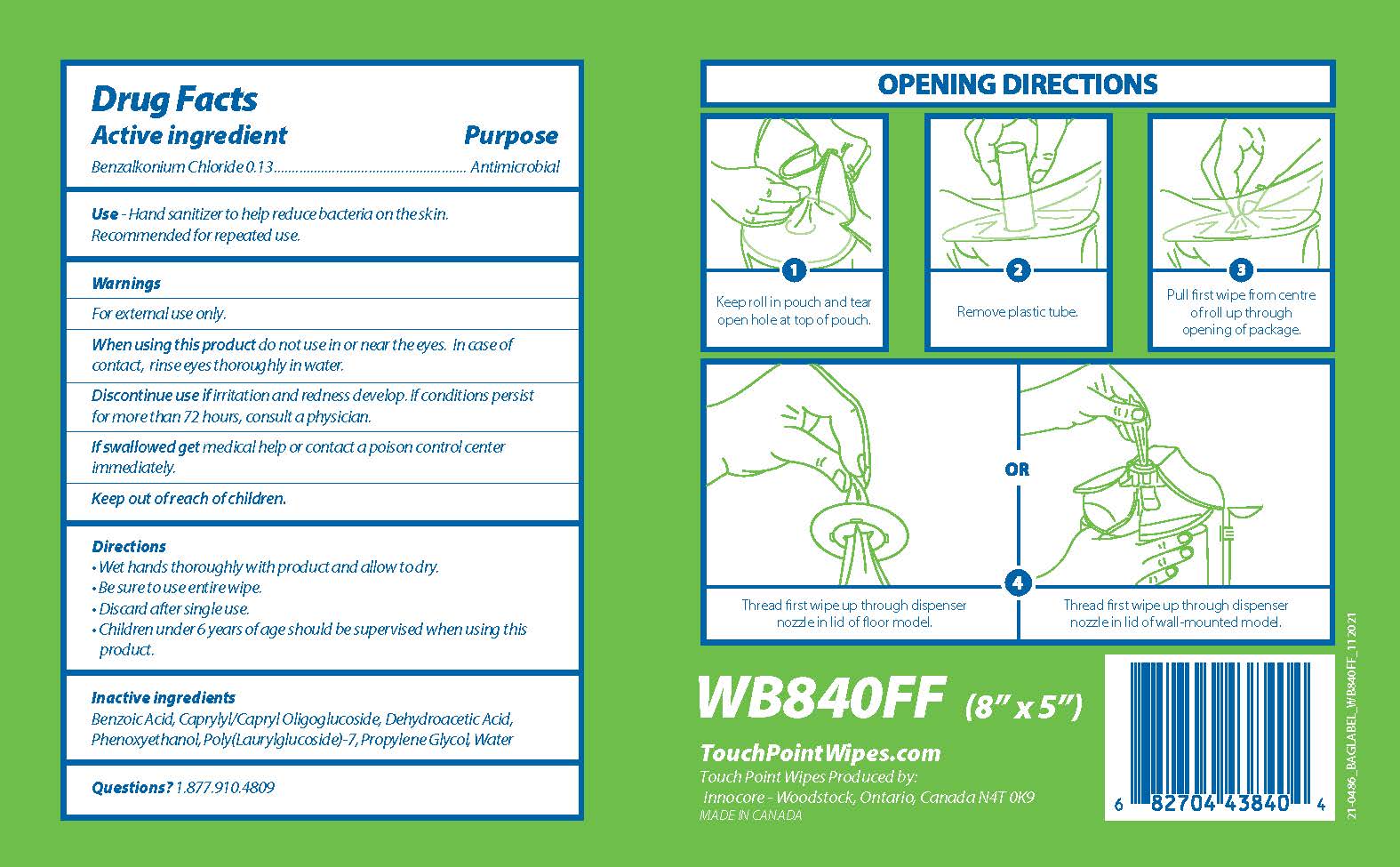
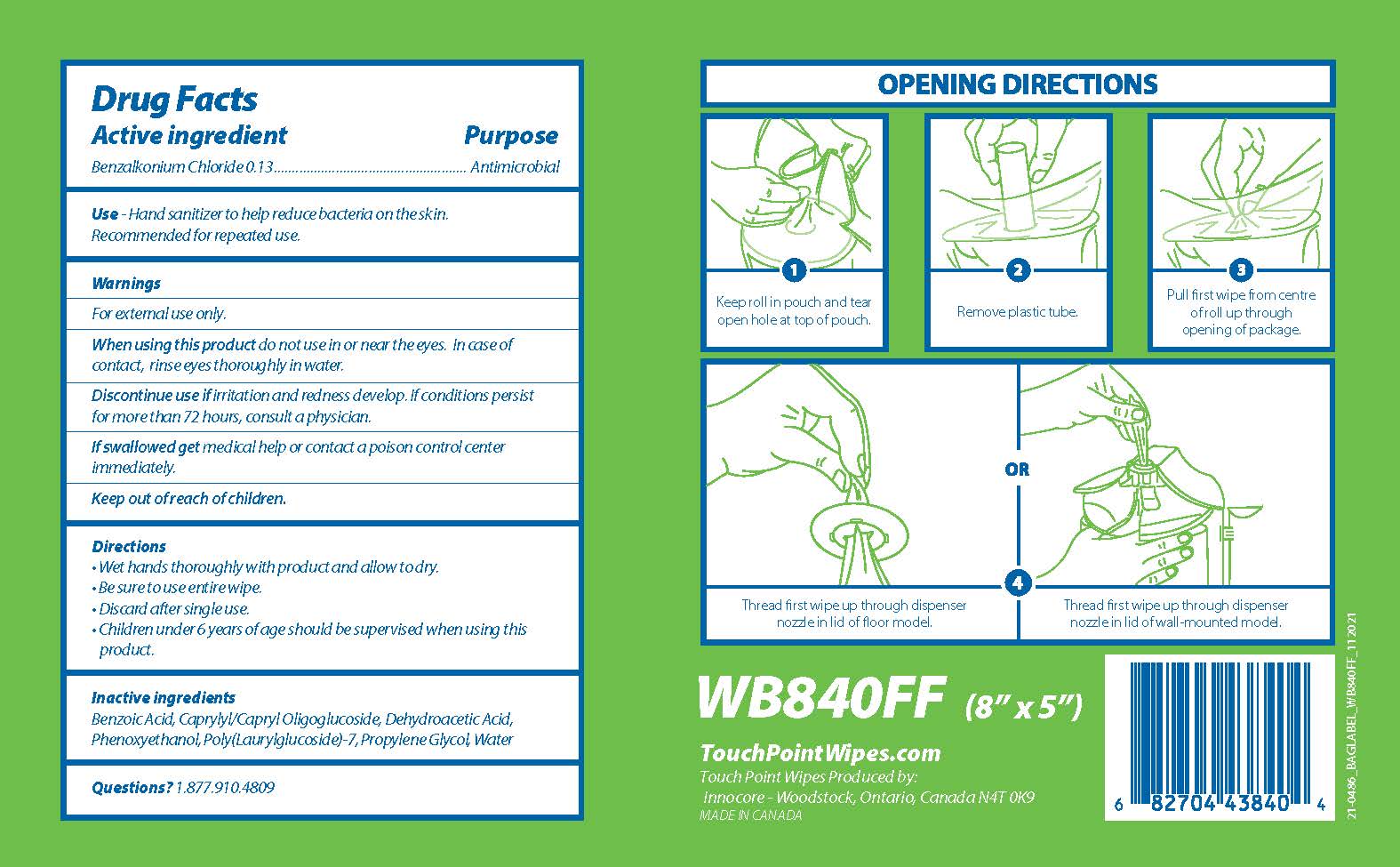
OPENING DIRECTIONS
1
Keep roll in pouch and tear
open hole at top of pouch
2
Remove plastic tube.
3
Pull first wipe from centre
of roll up through
opening of package
OR
4
Thread first wipe up through dispenser
nozzle in lid of floor model.
Thread first wipe up through dispenser
nozzle in lid of wall-mounted model.
WB840FF (8" x 5")
TouchPointWipes.com
Touch Point Wipes Produced by:
Innocore - Woodstock, Ontario, Canada N4T 0K9
MADE IN CANADA
-
INGREDIENTS AND APPEARANCE
TOUCHPOINT BIODEGRADABLE SANITIZING WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70924-004 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) POLY(LAURYLGLUCOSIDE)-7 (UNII: VB00RDE21R) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70924-004-02 2 in 1 BOX 01/01/2022 1 NDC:70924-004-01 840 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:70924-004-04 6 in 1 BOX 01/01/2022 2 NDC:70924-004-03 1000 in 1 CANISTER; Type 0: Not a Combination Product 3 NDC:70924-004-06 2 in 1 BOX 01/01/2022 3 NDC:70924-004-05 1200 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2022 Labeler - Innocore Sales & Marketing Inc (201152597)