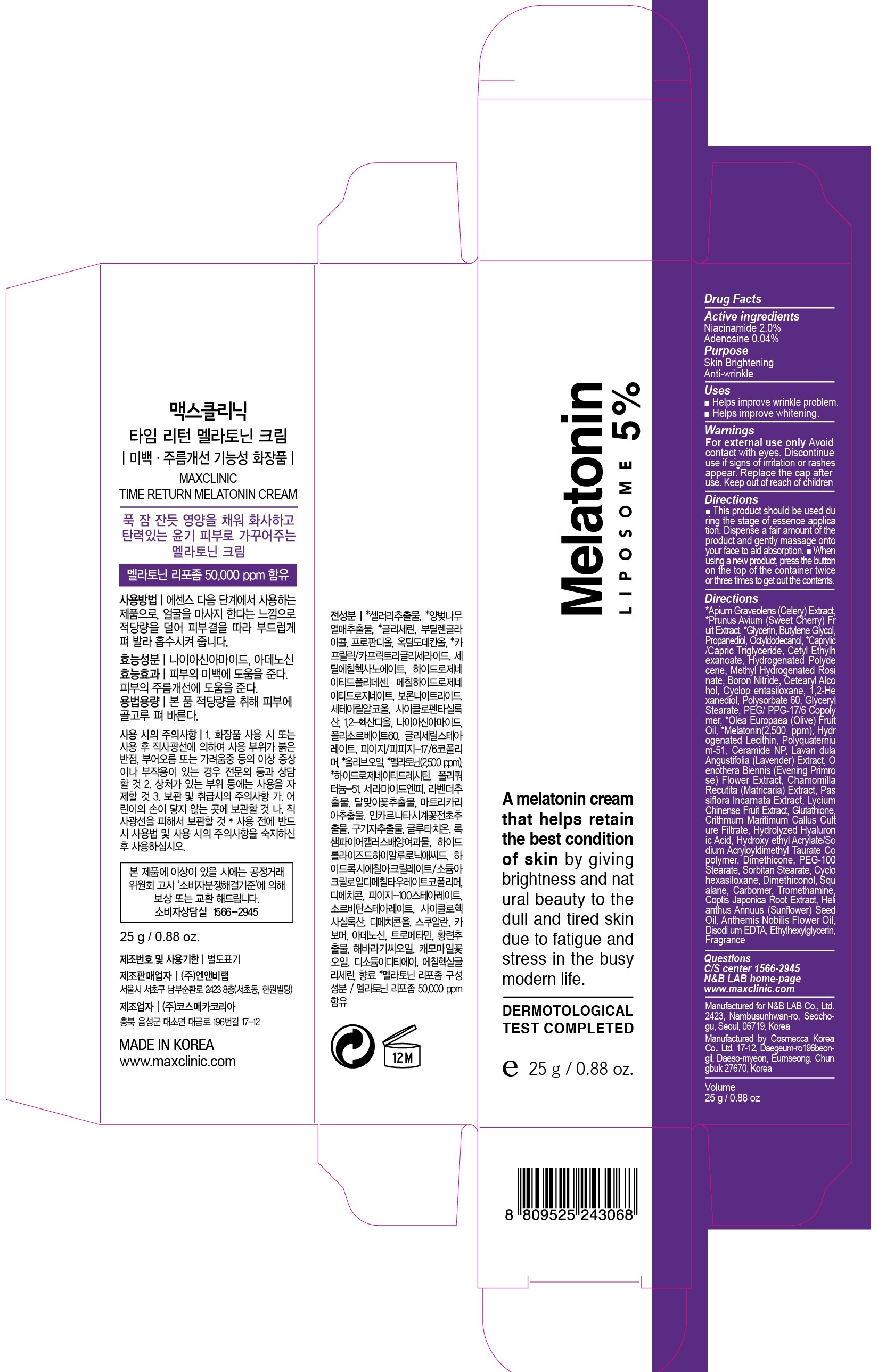
Label: TIME RETURN MELATONIN- niacinamide, adenosine patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 69058-610-01, 69058-610-02, 69058-610-03, 69058-610-04 - Packager: N&B LAB.Co.Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive ingredients: Niacinamide 2.0%, Adenosine 0.04%
-
INACTIVE INGREDIENTInactive ingredients: *Apium Graveolens (Celery) Extract, *Prunus Avium (Sweet Cherry) Fruit Extract, *Glycerin, Butylene Glycol, Propanediol, Octyldodecanol, *Caprylic/Capric Triglyceride, Cetyl ...
-
PURPOSEPurpose: Skin Brightening, Anti-wrinkle
-
WARNINGSWarnings: For external use only - Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear. Replace the cap after use. Keep out of reach of children
-
KEEP OUT OF REACH OF CHILDRENKEEP OUT OF REACH OF CHILDREN
-
UsesUses: ■ Helps improve wrinkle problem. ■ Helps improve whitening.
-
DirectionsDirections: ■ This product should be used during the stage of essence application. Dispense a fair amount of the product and gently massage onto your face to aid absorption. ■ When using a new ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - product image 50g
 ...
... -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - product image 25g
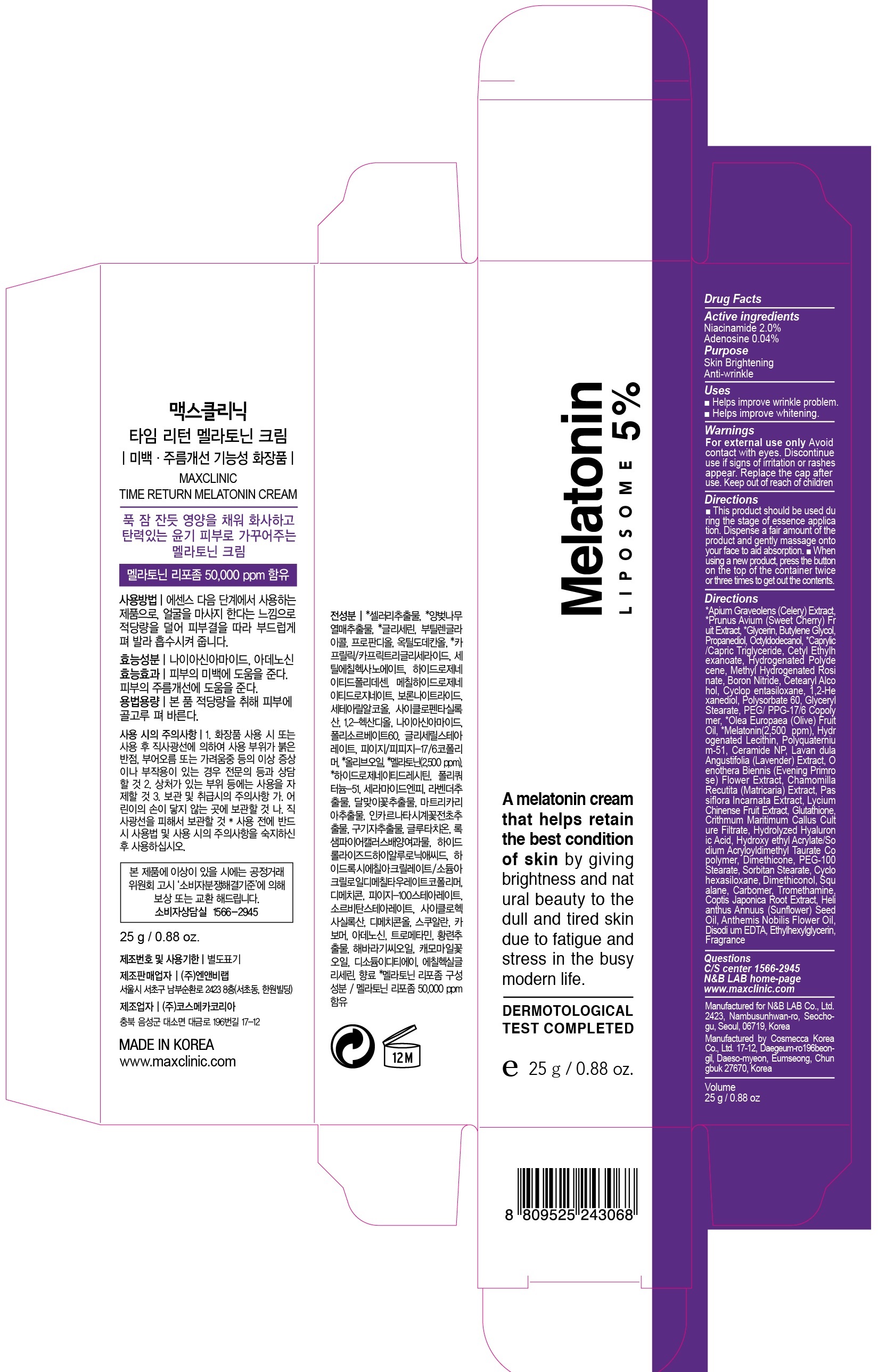 ...
... -
INGREDIENTS AND APPEARANCEProduct Information