Label: CEVIMELINE HYDROCHLORIDE capsule
- NDC Code(s): 16571-657-10
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Cevimeline is cis-2’-methylspiro {1-azabicyclo [2.2.2] octane-3, 5’ -[1,3] oxathiolane} hydrochloride, hydrate (2:1). Its empirical formula is C10H17NOS.HCl.1/2 H2O, and its structural formula is:
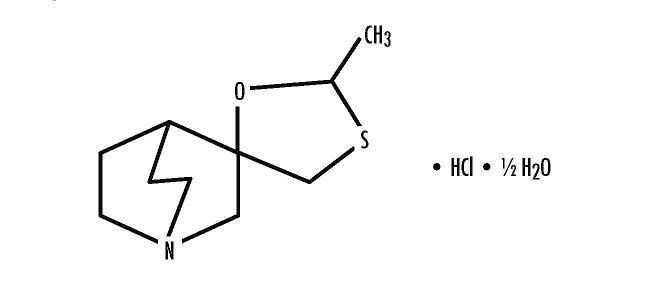
Cevimeline has a molecular weight of 244.79. It is a white to off white crystalline powder with a melting point range of 201 to 203°C. It is freely soluble in alcohol and chloroform, very soluble in water, and virtually insoluble in ether. The pH of a 1% solution ranges from 4.6 to 5.6. Inactive ingredients include lactose monohydrate, hydroxypropyl cellulose, and magnesium stearate.
Empty capsule shell consists of Titanium Dioxide and Gelatin. Ink used in the imprint is Black SW-9049 which contains Shellac, Dehydrated alcohol, Isopropyl Alcohol, Butyl Alcohol, Propylene Glycol, Purified Water, Strong Ammonia Solution, Potassium Hydroxide, and Black Iron Oxide.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
Cevimeline is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in sufficient dosage can increase secretion of exocrine glands, such as salivary and sweat glands and increase tone of the smooth muscle in the gastrointestinal and urinary tracts.Pharmacokinetics
Absorption: After administration of a single 30 mg capsule, cevimeline was rapidly absorbed with a mean time to peak concentration of 1.5 to 2 hours. No accumulation of active drug or its metabolites was observed following multiple dose administration. When administered with food, there is a decrease in the rate of absorption, with a fasting Tmax of 1.53 hours and a Tmax of 2.86 hours after a meal; the peak concentration is reduced by 17.3%. Single oral doses across the clinical dose range are dose proportional.Distribution: Cevimeline has a volume of distribution of approximately 6L/kg and is <20% bound to human plasma proteins. This suggests that cevimeline is extensively bound to tissues; however, the specific binding sites are unknown.
Metabolism: Isozymes CYP2D6 and CYP3A3/4 are responsible for the metabolism of cevimeline. After 24 hours, 86.7% of the dose was recovered (16.0% unchanged, 44.5% as cis and trans-sulfoxide, 22.3% of the dose as glucuronic acid conjugate and 4% of the dose as N-oxide of cevimeline). Approximately 8% of the trans-sulfoxide metabolite is then converted into the corresponding glucuronic acid conjugate and eliminated. Cevimeline did not inhibit cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4.
Excretion: The mean half-life of cevimeline is 5+/-1 hours. After 24 hours, 84% of a 30 mg dose of cevimeline was excreted in urine. After seven days, 97% of the dose was recovered in the urine and 0.5% was recovered in the feces.
Special Populations: The effects of renal impairment, hepatic impairment, or ethnicity on the pharmacokinetics of cevimeline have not been investigated.
Clinical Studies
Cevimeline has been shown to improve the symptoms of dry mouth in patients with Sjögren’s Syndrome.A 6-week, randomized, double blind, placebo-controlled study was conducted in 75 patients (10 men, 65 women) with a mean age of 53.6 years (range 33-75). The racial distribution was Caucasian 92%, Black 1% and other 7%. The effects of cevimeline at 30 mg tid (90 mg/day) and 60 mg tid (180 mg/day) were compared to those of placebo. Patients were evaluated by a measure called global improvement, which is defined as a response of “better” to the question, “Please rate the overall condition of your dry mouth now compared with how you felt before starting treatment in this study.” Patients also had the option of selecting “worse” or “no change” as answers. Seventy-six percent of the patients in the 30 mg tid group reported a global improvement in their dry mouth symptoms compared to 35% of the patients in the placebo group. This difference was statistically significant at p=0.0043. There was no evidence that patients in the 60 mg tid group had better global evaluation scores than the patients in the 30 mg tid group.
A 12-week, randomized, double-blind, placebo-controlled study was conducted in 197 patients (10 men, 187 women) with a mean age of 54.5 years (range 23-74). The racial distribution was Caucasian 91.4%, Black 3% and other 5.6%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. Statistically significant global improvement in the symptoms of dry mouth (p=0.0004) was seen for the 30 mg tid group compared to placebo, but not for the 15 mg group compared to placebo. Salivary flow showed statistically significant increases at both doses of cevimeline during the study compared to placebo.
A second 12-week, randomized, double-blind, placebo-controlled study was conducted in 212 patients (11 men, 201 women) with a mean age of 55.3 years (range 24-75). The racial distribution was Caucasian 88.7%, Black 1.9% and other 9.4%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. No statistically significant differences were noted in the patient global evaluations. However, there was a higher placebo response rate in this study compared to the aforementioned studies. The 30 mg tid group showed a statistically significant increase in salivary flow from pre- dose to post-dose compared to placebo (p=0.0017).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Cardiovascular Disease:
Cevimeline can potentially alter cardiac conduction and/or heart rate. Patients with significant cardiovascular disease may potentially be unable to compensate for transient changes in hemodynamics or rhythm induced by Cevimeline Hydrochloride capsules. Cevimeline Hydrochloride capsules should be used with caution and under close medical supervision in patients with a history of cardiovascular disease evidenced by angina pectoris or myocardial infarction.Pulmonary Disease:
Cevimeline can potentially increase airway resistance, bronchial smooth muscle tone, and bronchial secretions. Cevimeline should be administered with caution and with close medical supervision to patients with controlled asthma, chronic bronchitis, or chronic obstructive pulmonary disease.Ocular:
Ophthalmic formulations of muscarinic agonists have been reported to cause visual blurring which may result in decreased visual acuity, especially at night and in patients with central lens changes, and to cause impairment of depth perception. Caution should be advised while driving at night or performing hazardous activities in reduced lighting. -
PRECAUTIONS:
General:
Cevimeline toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory distress, gastrointestinal spasm, nausea, vomiting, diarrhea, atrioventricular block, tachycardia, bradycardia, hypotension, hypertension, shock, mental confusion, cardiac arrhythmia, and tremors.Cevimeline should be administered with caution to patients with a history of nephrolithiasis or cholelithiasis. Contractions of the gallbladder or biliary smooth muscle could precipitate complications such as cholecystitis, cholangitis and biliary obstruction. An increase in the ureteral smooth muscle tone could theoretically precipitate renal colic or ureteral reflux in patients with nephrolithiasis.
Information for Patients:
Patients should be informed that cevimeline may cause visual disturbances, especially at night, that could impair their ability to drive safely.If a patient sweats excessively while taking cevimeline, dehydration may develop. The patient should drink extra water and consult a health care provider.
Drug Interactions:
Cevimeline should be administered with caution to patients taking beta adrenergic antagonists, because of the possibility of conduction disturbances. Drugs with parasympathomimetic effects administered concurrently with cevimeline can be expected to have additive effects. Cevimeline might interfere with desirable antimuscarinic effects of drugs used concomitantly.Drugs which inhibit CYP2D6 and CYP3A3/4 also inhibit the metabolism of cevimeline. Cevimeline should be used with caution in individuals known or suspected to be deficient in CYP2D6 activity, based on previous experience, as they may be at a higher risk of adverse events. In an in vitro study, cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 were not inhibited by exposure to cevimeline.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Lifetime carcinogenicity studies were conducted in CD-1 mice and F-344 rats. A statistically significant increase in the incidence of adenocarcinomas of the uterus was observed in female rats that received cevimeline at a dosage of 100 mg/kg/day (approximately 8 times the maximum human exposure based on comparison of AUC data). No other significant differences in tumor incidence were observed in either mice or rats.Cevimeline exhibited no evidence of mutagenicity or clastogenicity in a battery of assays that included an Ames test, an in vitro chromosomal aberration study in mammalian cells, a mouse lymphoma study in L5178Y cells, or a micronucleus assay conducted in vivo in ICR mice.
Cevimeline did not adversely affect the reproductive performance or fertility of male Sprague- Dawley rats when administered for 63 days prior to mating and throughout the period of mating at dosages up to 45 mg/kg/day (approximately 5 times the maximum recommended dose for a 60 kg human following normalization of the data on the basis of body surface area estimates). Females that were treated with cevimeline at dosages up to 45 mg/kg/day from 14 days prior to mating through day seven of gestation exhibited a statistically significantly smaller number of implantations than did control animals.
Pregnancy:
Pregnancy Category C.
Cevimeline was associated with a reduction in the mean number of implantations when given to pregnant Sprague-Dawley rats from 14 days prior to mating through day seven of gestation at a dosage of 45 mg/kg/day (approximately 5 times the maximum recommended dose for a 60 kg human when compared on the basis of body surface area estimates). This effect may have been secondary to maternal toxicity. There are no adequate and well-controlled studies in pregnant women. Cevimeline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Nursing Mothers:
It is not known whether this drug is secreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Cevimeline Hydrochloride capsules, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.Geriatric Use:
Although clinical studies of cevimeline included subjects over the age of 65, the numbers were not sufficient to determine whether they respond differently from younger subjects. Special care should be exercised when cevimeline treatment is initiated in an elderly patient, considering the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug
therapy in the elderly. -
ADVERSE REACTIONS
Cevimeline was administered to 1777 patients during clinical trials worldwide, including Sjögren’s patients and patients with other conditions. In placebo-controlled Sjögren’s studies in the U.S., 320 patients received cevimeline doses ranging from 15 mg tid to 60 mg tid, of whom 93% were women and 7% were men. Demographic distribution was 90% Caucasian, 5% Hispanic, 3% Black and 2% of other origin. In these studies, 14.6% of patients discontinued treatment with cevimeline due to adverse events.
The following adverse events associated with muscarinic agonism were observed in the clinical trials of cevimeline in Sjögren’s syndrome patients:- *
- n is the total number of patients exposed to the dose at any time during the study.
Adverse Event Cevimeline 30 mg (tid)
n*= 533Placebo (tid)
n*= 164Excessive sweating 18.7% 2.4% Nausea 13.8% 7.9% Rhinitis 11.2% 5.4% Diarrhea 10.3% 10.3% Excessive salivation 2.2% 0.6% Urinary frequency 0.9% 1.8% Asthenia 0.5% 0.0% Flushing 0.3% 0.6% Polyuria 0.1% 0.6% In addition, the following adverse events (≥3% incidence) were reported in the Sjögren’s clinical trials:
- *
- n is the total number of patients exposed to the dose at any time during the study.
Adverse Event Cevimeline 30 mg (tid)
n*=533Placebo (tid)
n*=164Headache 14.4% 20.1% Sinusitis 12.3% 10.9% Upper respiratory Tract Infection 11.4% 9.1% Dyspepsia 7.8% 8.5% Abdominal pain 7.6% 6.7% Urinary Tract Infection 6.1% 3.0% Coughing 6.1% 3.0% Pharyngitis 5.2% 5.4% Vomiting 4.6% 2.4% Injury 4.5% 2.4% Back pain 4.5% 4.2% Rash 4.3% 6.0% Conjunctivitis 4.3% 3.6% Dizziness 4.1% 7.3% Bronchitis 4.1% 1.2% Arthralgia 3.7% 1.8% Surgical intervention 3.3% 3.0% Fatigue 3.3% 1.2% Pain 3.3% 3.0% Skeletal pain 2.8% 1.8% Insomnia 2.4% 1.2% Hot flushes 2.4% 0.0% Rigors 1.3% 1.2% Anxiety 1.3% 1.2% The following events were reported in Sjögren’s patients at incidences of <3% and ≥1%: constipation, tremor, abnormal vision, hypertonia, peripheral edema, chest pain, myalgia, fever, anorexia, eye pain, earache, dry mouth, vertigo, salivary gland pain, pruritus, influenza- like symptoms, eye infection, post-operative pain, vaginitis, skin disorder, depression, hiccup, hyporeflexia, infection, fungal infection, sialoadenitis, otitis media, erythematous rash, pneumonia, edema, salivary gland enlargement, allergy, gastroesophageal reflux, eye abnormality, migraine, tooth disorder, epistaxis, flatulence, toothache, ulcerative stomatitis, anemia, hypoesthesia, cystitis, leg cramps, abscess, eructation, moniliasis, palpitation, increased amylase, xerophthalmia, allergic reaction.
The following events were reported rarely in treated Sjögren’s patients (<1%): Causal relation is unknown:
Body as a Whole Disorders: aggravated allergy, precordial chest pain, abnormal crying, hematoma, leg pain, edema, periorbital edema, activated pain trauma, pallor, changed sensation temperature, weight decrease, weight increase, choking, mouth edema, syncope, malaise, face edema, substernal chest pain.
Cardiovascular Disorders: abnormal ECG, heart disorder, heart murmur, aggravated hyper- tension, hypotension, arrhythmia, extrasystoles, t wave inversion, tachycardia, supraventricular tachycardia, angina pectoris, myocardial infarction, pericarditis, pulmonary embolism, peripheral ischemia, superficial phlebitis, purpura, deep thrombophlebitis, vascular disorder, vasculitis, hypertension.
Digestive Disorders: appendicitis, increased appetite, ulcerative colitis, diverticulitis, duodenitis, dysphagia, enterocolitis, gastric ulcer, gastritis, gastroenteritis, gastrointestinal hemorrhage, gingivitis, glossitis, rectum hemorrhage, hemorrhoids, ileus, irritable bowel syndrome, melena, mucositis, esophageal stricture, esophagitis, oral hemorrhage, peptic ulcer, periodontal destruction, rectal disorder, stomatitis, tenesmus, tongue discoloration, tongue disorder, geographic tongue, tongue ulceration, dental caries.
Endocrine Disorders: increased glucocorticoids, goiter, hypothyroidism.
Hematologic Disorders: thrombocytopenic purpura, thrombocythemia, thrombocytopenia, hypochromic anemia, eosinophilia, granulocytopenia, leucopenia, leukocytosis, cervical lymphadenopathy, lymphadenopathy.
Liver and Biliary System Disorders: cholelithiasis, increased gamma-glutamyl transferase, increased hepatic enzymes, abnormal hepatic function, viral hepatitis, increased serum glutamate oxaloacetic transaminase (SGOT) (also called AST-aspartate aminotransferase), increased serum glutamate pyruvate transaminase (SGPT) (also called ALT-alanine amino- transferase).
Metabolic and Nutritional Disorders: dehydration, diabetes mellitus, hypercalcemia, hypercholesterolemia, hyperglycemia, hyperlipemia, hypertriglyceridemia, hyperuricemia, hypoglycemia, hypokalemia, hyponatremia, thirst.
Musculoskeletal Disorders: arthritis, aggravated arthritis, arthropathy, femoral head avascular necrosis, bone disorder, bursitis, costochondritis, plantar fasciitis, muscle weakness, osteomyelitis, osteoporosis, synovitis, tendinitis, tenosynovitis.
Neoplasms: basal cell carcinoma, squamous carcinoma.
Nervous Disorders: carpal tunnel syndrome, coma, abnormal coordination, dysesthesia, dyskinesia, dysphonia, aggravated multiple sclerosis, involuntary muscle contractions, neuralgia, neuropathy, paresthesia, speech disorder, agitation, confusion, depersonalization, aggravated depression, abnormal dreaming, emotional lability, manic reaction, paroniria, somnolence, abnormal thinking, hyperkinesia, hallucination.
Miscellaneous Disorders: fall, food poisoning, heat stroke, joint dislocation, post-operative hemorrhage.
Resistance Mechanism Disorders: cellulitis, herpes simplex, herpes zoster, bacterial infection, viral infection, genital moniliasis, sepsis.
Respiratory Disorders: asthma, bronchospasm, chronic obstructive airway disease, dyspnea, hemoptysis, laryngitis, nasal ulcer, pleural effusion, pleurisy, pulmonary congestion, pulmonary fibrosis, respiratory disorder.
Rheumatologic Disorders: aggravated rheumatoid arthritis, lupus erythematosus rash, lupus erythematosus syndrome.
Skin and Appendages Disorders: acne, alopecia, burn, dermatitis, contact dermatitis, lichenoid dermatitis, eczema, furunculosis, hyperkeratosis, lichen planus, nail discoloration, nail disorder, onychia, onychomycosis, paronychia, photosensitivity reaction, rosacea, scleroderma, seborrhea, skin discoloration, dry skin, skin exfoliation, skin hypertrophy, skin ulceration, urticaria, verruca, bullous eruption, cold clammy skin.
Special Senses Disorders: deafness, decreased hearing, motion sickness, parosmia, taste perversion, blepharitis, cataract, corneal opacity, corneal ulceration, diplopia, glaucoma, anterior chamber eye hemorrhage, keratitis, keratoconjunctivitis, mydriasis, myopia, photopsia, retinal deposits, retinal disorder, scleritis, vitreous detachment, tinnitus.
Urogenital Disorders: epididymitis, prostatic disorder, abnormal sexual function, amenorrhea, female breast neoplasm, malignant female breast neoplasm, female breast pain, positive cervical smear test, dysmenorrhea, endometrial disorder, intermenstrual bleeding, leukorrhea, menorrhagia, menstrual disorder, ovarian cyst, ovarian disorder, genital pruritus, uterine hemorrhage, vaginal hemorrhage, atrophic vaginitis, albuminuria, bladder discomfort, increased blood urea nitrogen, dysuria, hematuria, micturition disorder, nephrosis, nocturia, increased nonprotein nitrogen, pyelonephritis, renal calculus, abnormal renal function, renal pain, strangury, urethral disorder, abnormal urine, urinary incontinence, decreased urine flow, pyuria.
In one subject with lupus erythematosus receiving concomitant multiple drug therapy, a highly elevated ALT level was noted after the fourth week of cevimeline therapy. In two other subjects receiving cevimeline in the clinical trials, very high AST levels were noted. The significance of these findings is unknown.
Additional adverse events (relationship unknown) which occurred in other clinical studies (patient population different from Sjögren’s patients) are as follows:
cholinergic syndrome, blood pressure fluctuation, cardiomegaly, postural hypotension, aphasia, convulsions, abnormal gait, hyperesthesia, paralysis, abnormal sexual function, enlarged abdomen, change in bowel habits, gum hyperplasia, intestinal obstruction, bundle branch block, increased creatine phosphokinase, electrolyte abnormality, glycosuria, gout, hyper- kalemia, hyperproteinemia, increased lactic dehydrogenase (LDH), increased alkaline phosphatase, failure to thrive, abnormal platelets, aggressive reaction, amnesia, apathy, delirium, delusion, dementia, illusion, impotence, neurosis, paranoid reaction, personality disorder, hyperhemoglobinemia, apnea, atelectasis, yawning, oliguria, urinary retention, distended vein, lymphocytosis.
The following adverse reaction has been identified during post-approval use of Cevimeline Hydrochloride. Because post-marketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
MANAGEMENT OF OVERDOSE
Management of the signs and symptoms of acute overdosage should be handled in a manner consistent with that indicated for other muscarinic agonists: general supportive measures should be instituted. If medically indicated, atropine, an anti-cholinergic agent, may be of value as an antidote for emergency use in patients who have had an overdose of cevimeline. If medically indicated, epinephrine may also be of value in the presence of severe cardiovascular depression or bronchoconstriction. It is not known if cevimeline is dialyzable.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Cevimeline Hydrochloride Capsules, 30 mg are available as white, hard gelatin capsules containing 30 mg of Cevimeline Hydrochloride. Cevimeline Hydrochloride Capsules are size 3 hard gelatin capsules with an opaque white cap and body. Cevimeline Hydrochloride capsules are imprinted with P 657 on both cap and body in black ink. Cevimeline Hydrochloride is supplied in child resistant bottles of:
100 capsules (NDC: 16571-657-10)
Store at 25°C (77°F) excursions permitted to 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature].Rx only
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Rev. 07/24
551604
PIR65710-02 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CEVIMELINE HYDROCHLORIDE
cevimeline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-657 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cevimeline Hydrochloride (UNII: P81Q6V85NP) (CEVIMELINE - UNII:K9V0CDQ56E) CEVIMELINE HYDROCHLORIDE ANHYDROUS 30 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) hydroxypropyl cellulose (UNII: RFW2ET671P) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color WHITE (opaque white cap and body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code P;657 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-657-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203775 06/17/2014 Labeler - Rising Pharma Holdings, Inc. (116880195) Establishment Name Address ID/FEI Business Operations Ingenus Pharmaceuticals NJ, LLC 964680206 MANUFACTURE(16571-657)