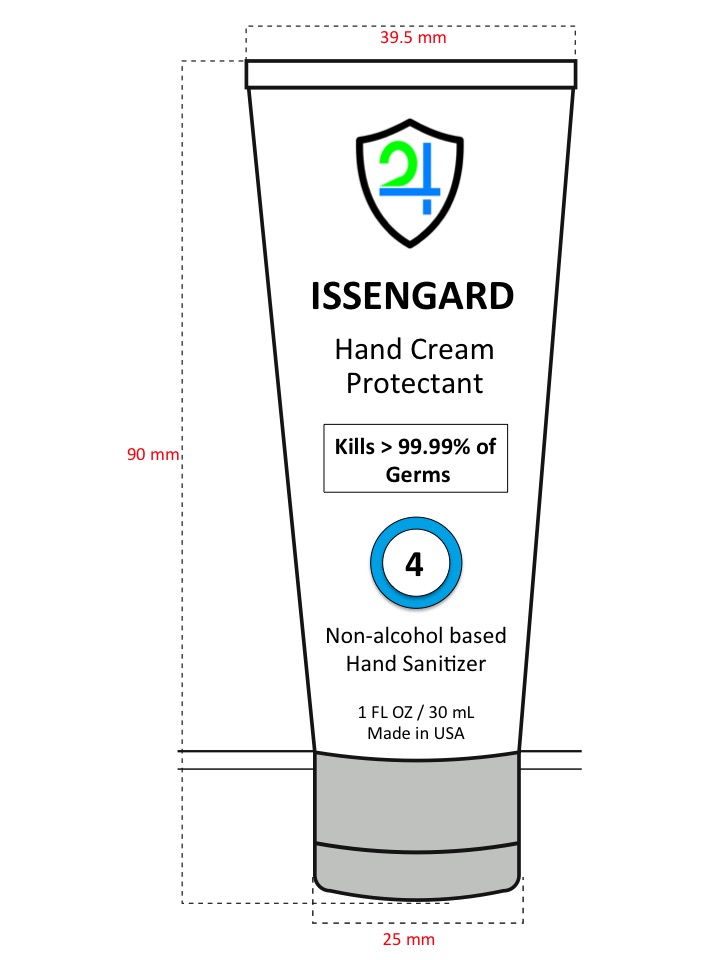
Label: ISSENGARD 24 HAND SANITIZER 3 OZ- benzalkonium chloride cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 81495-112-01 - Packager: CLEAR LAKE RESEARCH, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
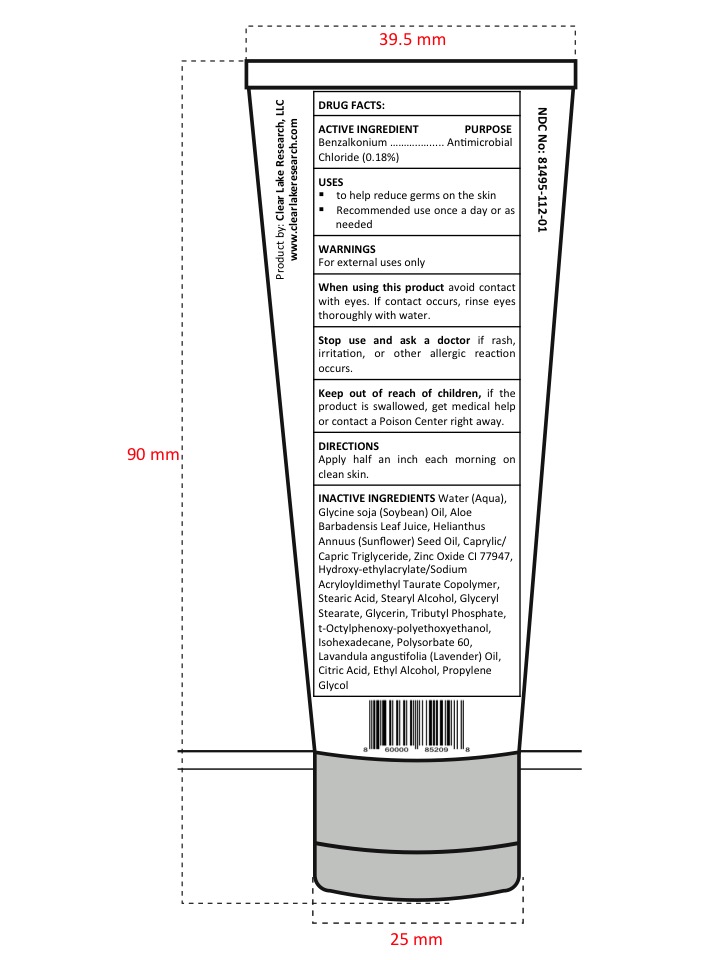
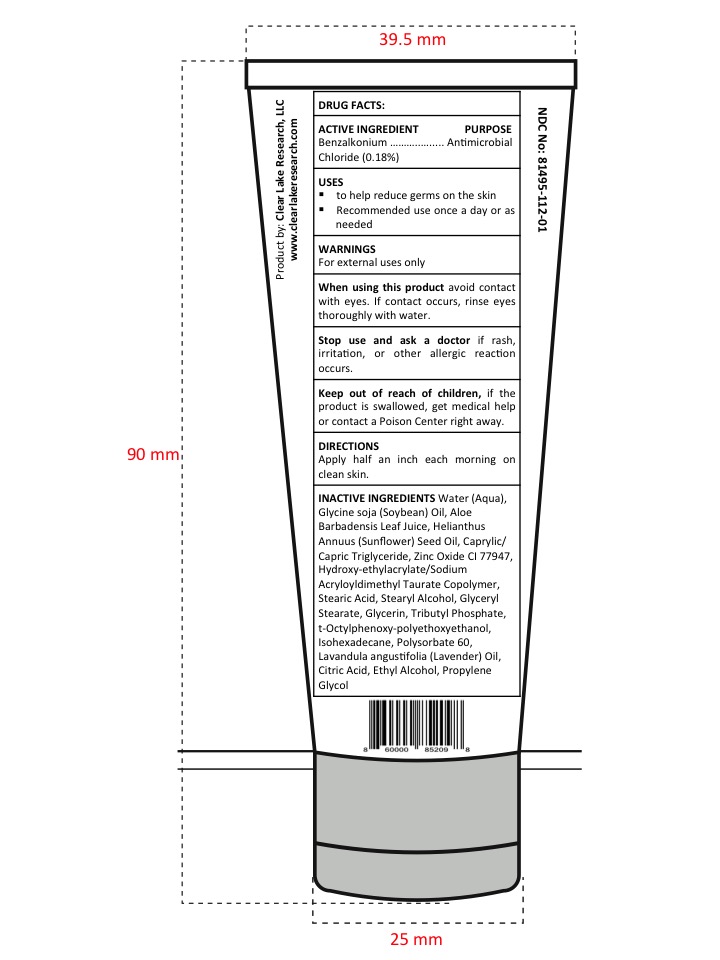
- Active Ingredient
- Purpose section
- Warnings
- Drug Facts
- When using this product
- Stop use and ask a doctor
- Keep Out of Reach of Children
- Uses
- Directions
-
Inactive Ingredients
Water (Aqua), Glycine soja (Soybean) Oil, Aloe Barbadensis Leaf Juice, Helianthus Annuus (Sunflower) Seed Oil, Caprylic/Capric Triglyceride, Tributyl Phosphate, Glycerin, t-Octylphenoxy-polyethoxyethanol, Hydroxy-ethylacrylate/Sodium Acryloyldimethyl Taurate, Copolymer, Isohexadecane, Polysorbate 60, Glyceryl Stearate, Stearic Acid, Stearyl Alcohol, Lavandula angustifolia (Lavender) Oil, Propylene Glycol, Ethyl Alcohol, Zinc Oxide CI 77947
- Principal Display Panel - 30 mL
-
INGREDIENTS AND APPEARANCE
ISSENGARD 24 HAND SANITIZER 3 OZ
benzalkonium chloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81495-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.18 mg in 100 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 60 (UNII: CAL22UVI4M) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) ISOHEXADECANE (UNII: 918X1OUF1E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ZINC OXIDE (UNII: SOI2LOH54Z) GLYCERIN (UNII: PDC6A3C0OX) OCTOXYNOL-1 (UNII: 20CAX7IO75) ALCOHOL (UNII: 3K9958V90M) SOYBEAN OIL (UNII: 241ATL177A) LAVENDER OIL (UNII: ZBP1YXW0H8) SUNFLOWER OIL (UNII: 3W1JG795YI) TRI-N-BUTYL PHOSPHATE (UNII: 95UAS8YAF5) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) STEARIC ACID (UNII: 4ELV7Z65AP) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81495-112-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 01/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/03/2022 Labeler - CLEAR LAKE RESEARCH, LLC (077493647) Establishment Name Address ID/FEI Business Operations Clear Lake Research, LLC 077493647 label(81495-112)