Label: KATE SUMMERVILLE ERADIKATE- sulfur cream
- NDC Code(s): 11090-263-02
- Packager: Beauty Manufacturing Solutions Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 11, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
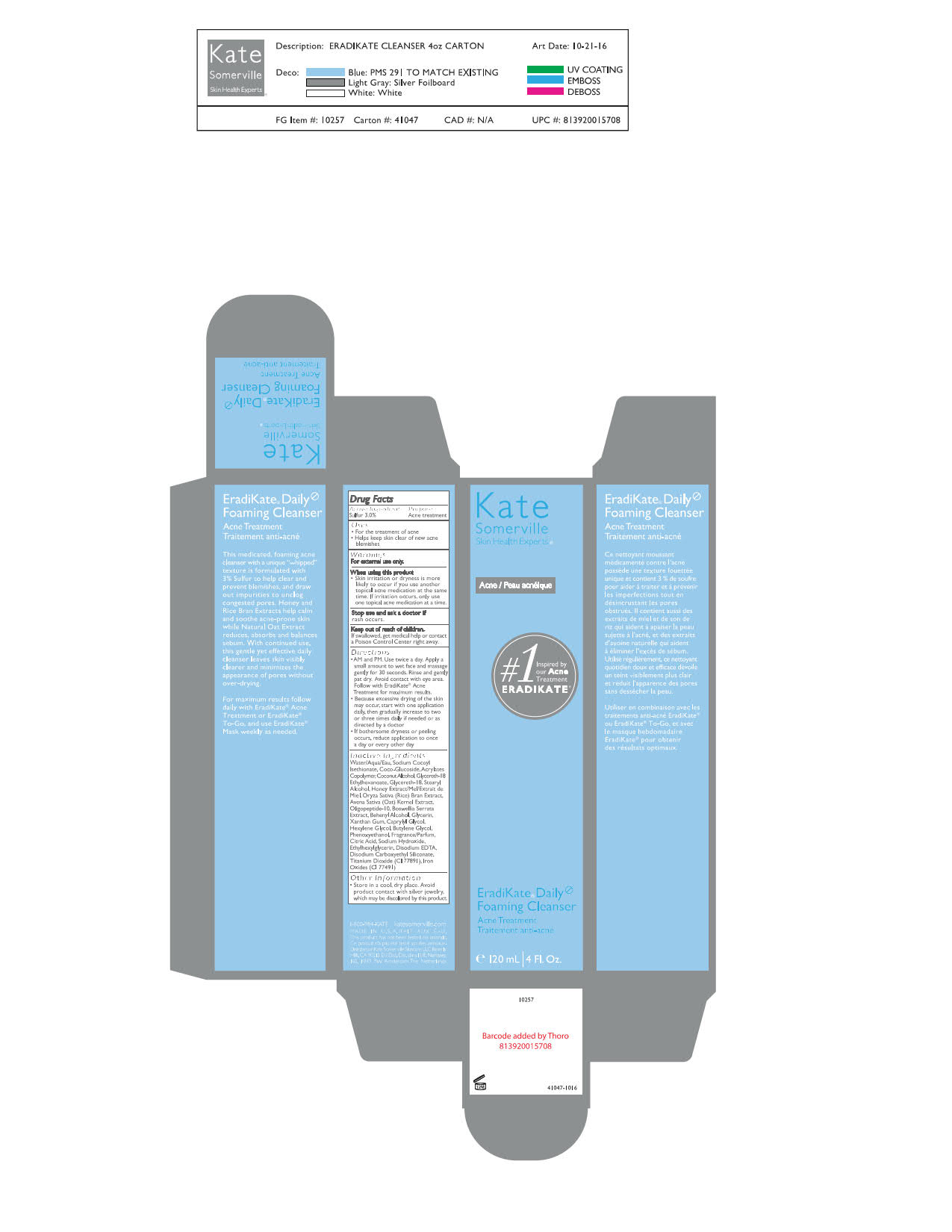
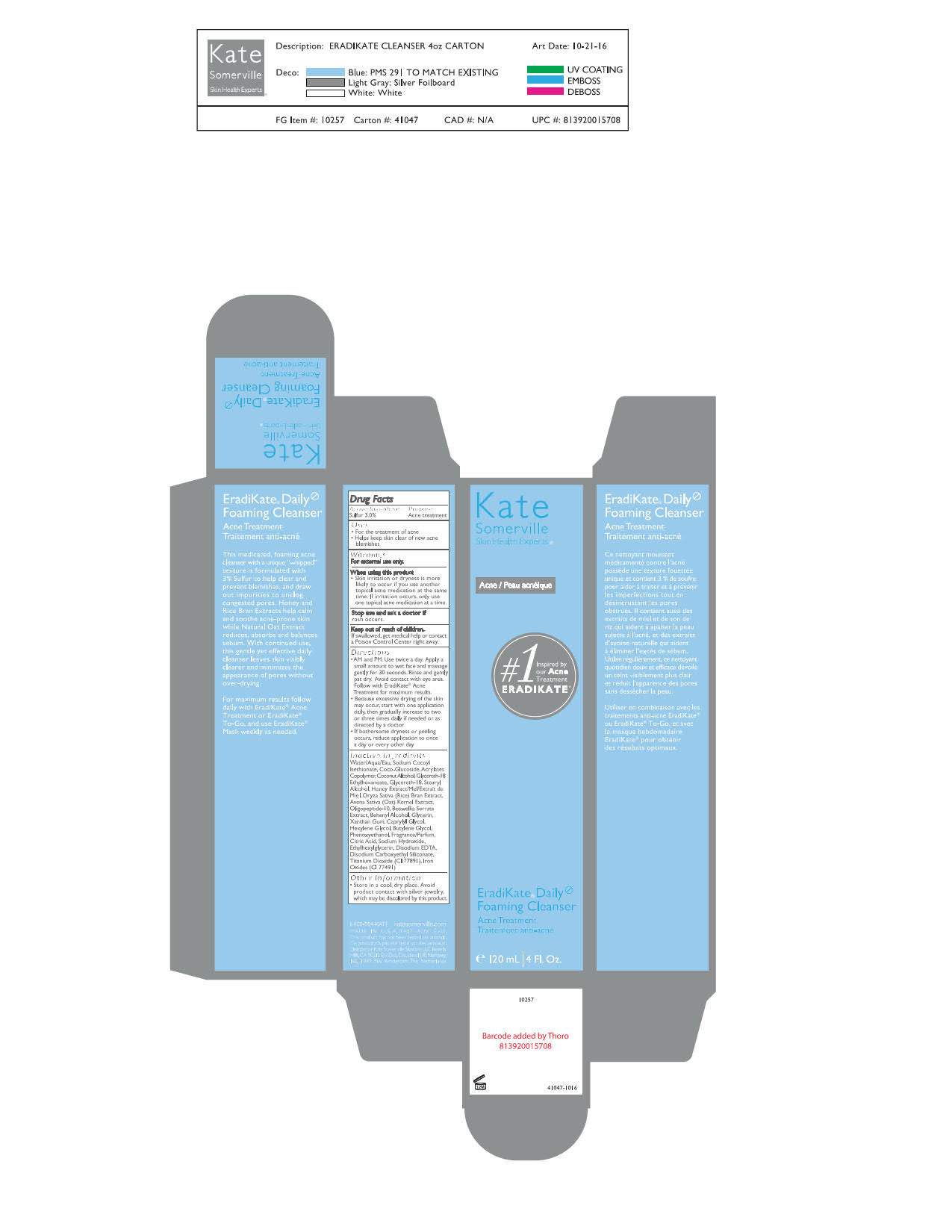
- AM and PM. Use twice a day. Apply a small amount to wet face and massage gently for 30 seconds. Rinse and gently pat dry. Avoid contact with eye area. Follow with EradiKate Acne Treatment for maximum results.
- Because of excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Aqua/Water/Eau, Sodium Cocoyl Isethionate, Coco-Glucoside, Acrylates Copolymer, Coconut Alcohol, Glycereth-18 Ethylhexanoate, Glycereth-18, Stearyl Alcohol, Honey Extract, Oryza Sativa (Rice) Bran Extract, Avena Sativa (Oat) Kernel Extract, Oligopeptide-10, Boswellia Serrata Extract, Behenyl Alcohol, Glycerin, Xanthan Gum, Caprylyl Glycol, Hexylene Glycol, Butylene Glycol, Phenoxyethanol, Fragrance (Parfum), Citric Acid, Sodium Hydroxide, Ethylhexylglycerin, Disodium EDTA, Disodium Carboxyethyl Siliconate, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491)
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KATE SUMMERVILLE ERADIKATE
sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-263 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 3 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) SODIUM CHLORIDE (UNII: 451W47IQ8X) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) DOCOSANOL (UNII: 9G1OE216XY) GLYCERETH-18 ETHYLHEXANOATE (UNII: IWS58C6V2Y) OAT BRAN (UNII: KQX236OK4U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) XANTHAN GUM (UNII: TTV12P4NEE) DISODIUM CARBOXYETHYL SILICONATE (UNII: 4U4C79679G) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HONEY (UNII: Y9H1V576FH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) COCONUT ALCOHOL (UNII: 13F4MW8Y9K) RICE BRAN OIL (UNII: LZO6K1506A) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERETH-18 (UNII: SA5E43C17C) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-263-02 1 in 1 BOX 02/01/2022 1 120 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 02/01/2022 Labeler - Beauty Manufacturing Solutions Corp. (783200723) Registrant - Beauty Manufacturing Solutions Corp. (783200723) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(11090-263)