Label: TECHNETIUM TC 99M MERTIATIDE injection, powder, lyophilized, for solution
- NDC Code(s): 65174-261-01, 65174-261-05
- Packager: Jubilant DraxImage Inc., dba Jubilant Radiopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MERTIATIDE safely and effectively. See full prescribing information for Kit for the preparation of Technetium Tc 99m Mertiatide.
KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MERTIATIDE, for intravenous use
Initial U.S. Approval: 1990INDICATIONS AND USAGE
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection, after radiolabeling with technetium-99m, is a radioactive diagnostic agent indicated for use in the diagnosis of congenital and acquired renal abnormalities, renal failure, urinary tract obstruction, and calculi in adults and pediatric patients aged 30 days and older. The product is a diagnostic aid in providing renal function, split function, renal angiograms, and renogram curves for whole kidney and renal cortex. (1)
DOSAGE AND ADMINISTRATION
- Instruct patients to drink a sufficient amount of water before administration and continue to drink and to void frequently following administration. (2.2)
- Recommended dose in adult patients:185 MBq to 370 MBq (5 mCi to 10 mCi) as an intravenous bolus injection. (2.3)
- Recommended dose in pediatric patients aged 30 days and older: 2.6 MBq/kg to 5.2 MBq/kg (0.07 mCi/kg to 0.14 mCi/kg) with a minimum dose of 37 MBq (1 mCi) as an intravenous bolus injection. (2.3)
- See Full Prescribing Information for radiation safety, drug preparation, administration, and radiation dosimetry information. (2.1, 2.4, 2.5, 2.6, 2.7)
DOSAGE FORMS AND STRENGTHS
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection: 1 mg betiatide as a lyophilized powder in 10 ml glass multiple-dose vial. Upon radiolabeling with technetium-99m, it contains up to 3,700 MBq (100 mCi) technetium Tc 99m mertiatide in approximately 10 mL volume at calibration time. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Radiation risks: Ensure safe handling to minimize radiation exposure to patients and healthcare providers. (5.1)
ADVERSE REACTIONS
Reported adverse reactions are: tachycardia, nausea, vomiting, shaking chills, fever, seizure, wheezing, dyspnea, itching, rash, and elevation in blood pressure. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Jubilant DraxImage Inc. at 1-888-633-5343 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breast milk for at least 24 hours after Technetium Tc99m Mertiatide administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
2.2 Patient Preparation
2.3 Recommended Dosage
2.4 Drug Preparation and Handling
2.5 Determination of Radiochemical Purity of Technetium Tc 99m Mertiatide Injection
2.6 Administration Instructions
2.7 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection, after radiolabeling with technetium-99m, is indicated for use in the diagnosis of congenital and acquired renal abnormalities, renal failure, urinary tract obstruction, and calculi in adults and pediatric patients aged 30 days and older. The product is a diagnostic aid in providing renal function, split function, renal angiograms, and renogram curves for whole kidney and renal cortex.
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
After radiolabeling, the kit produces Technetium Tc 99m Mertiatide Injection. Handle Technetium Tc 99m Mertiatide Injection with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.1)]. Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling Technetium Tc 99m Mertiatide Injection.
Radiopharmaceuticals should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Patient Preparation
Instruct patients to drink a sufficient amount of water to ensure adequate hydration prior to administration of Technetium Tc 99m Mertiatide Injection and to continue to drink and void frequently following administration to reduce radiation exposure.
2.3 Recommended Dosage
Adult
The recommended dose in adult patients is 185 MBq to 370 MBq (5 mCi to 10 mCi) as an intravenous bolus injection.Pediatric
The recommended dose in pediatric patients aged 30 days and older is 2.6 MBq/kg to 5.2 MBq/kg (0.07 mCi/kg to 0.14 mCi/kg) of actual body weight with a minimum dose of 37 MBq (1 mCi) as an intravenous bolus injection.2.4 Drug Preparation and Handling
Procedural Precautions
- Contents of the vials are intended only for use in the preparation of Technetium Tc 99m Mertiatide Injection, and are NOT to be administered directly to the patient.
- Do not use Sodium Pertechnetate Tc 99m Injection USP more than 6 hours after its elution from the generator.
- Use aseptic techniques for all transfers and vial stopper entries.
- Wear waterproof gloves during the procedure, particularly during the heating of the sample with boiling water.
- Use suitable shielding to reduce radiation exposure. Use one lead shield for incubation with a water bath and another lead shield for dispensing as described in the Procedure for the Preparation of Technetium Tc 99m Mertiatide Injection section below. Make all transfers of sodium pertechnetate Tc 99m solution during the preparation procedure with an adequately shielded syringe.
Procedure for the Preparation of Technetium Tc 99m Mertiatide Injection
- Prepare a rolling boil water bath containing a lead incubation vial shield with openings cut in it to allow the water to circulate through the shield. Orient the openings of the lead incubation shield to prevent radiation leakage.
Note: The vial should be in direct contact with the rolling boil water of the bath, and the level of the bath must be at least even with the level of the contents of the vial. - Ensure the temperature of the water bath used for heating the reaction vial is at boiling temperature and the temperature of the lead incubation vial shield is equilibrated to the temperature of the water bath before proceeding with the preparation procedure below.
- Place the reaction vial in a lead dispensing shield fitted with a lid and with a minimum wall thickness of 0.32 cm.
- Swab the rubber stopper of the reaction vial with an appropriate antiseptic wipe. Insert a filter-containing venting needle (provided) through the vial stopper.
- Add 4 mL to 10 mL of Sodium Pertechnetate Tc 99m Injection USP containing 740 MBq to 3,700 MBq (20 mCi to 100 mCi) into the vial using a sterile syringe. If necessary, dilute the Sodium Pertechnetate Tc 99m Injection USP with 0.9% Sodium Chloride Injection USP to the desired concentration prior to addition to the vial.
- Immediately following the addition of Sodium Pertechnetate Tc 99m Injection USP to the reaction vial, withdraw the syringe plunger to a volume of 2 mL, thus removing 2 mL of argon gas, which adds 2 mL of filtered air to the vial to oxidize excess stannous ion and to prevent the formation of technetium-99m labeled impurities.
Note:The sample vial must be placed in boiling water bath within 5 minutes of the addition of Sodium Pertechnetate Tc 99m solution. - Remove both needles from the vial.
- Invert the reaction vial several times to obtain complete mixing, prior to heating.
- Immediately transfer the reaction vial to the water bath. Place it inside the lead incubation shield which has been equilibrated to the temperature of the boiling water bath. The reaction vial should be in direct contact with the rolling boil water of the bath, and the level of the water must be at least even with the level of the contents of the vial. Incubate the reaction vial for 10 minutes.
- Remove the reaction vial from the boiling water bath and place it in the lead dispensing shield. Allow the contents of the vial to cool for approximately 15 minutes.
- Using proper shielding, visually inspect the vial contents. The solution should be clear and free of particulate matter. If not, do not use the preparation.
- Assay the reaction vial using a suitable radioactivity calibration system. Record the date, time, total activity, volume, and concentration on the radioassay information label and affix the label to the lead dispensing shield.
- Test the radiochemical purity of the prepared Technetium Tc 99m Mertiatide Injection according to the recommended method [see Dosage and Administration (2.5)]. If the radiochemical purity is less than 90%, do not use the product.
- Test the radiochemical impurities of the prepared Technetium Tc 99m Mertiatide Injection according to the recommended method [see Dosage and Administration (2.5)]. If the sum of the hydrophilic impurities and non-elutable impurities is more than 10%, do not use the product.
- Use the Technetium Tc 99m Mertiatide Injection within 6 hours of radiolabeling and store it at room temperature 15°C to 30°C (59°F to 86°F) until use.
- Handle and dispose unused radioactive material in accordance with applicable regulations.
2.5 Determination of Radiochemical Purity of Technetium Tc 99m Mertiatide Injection
Required Materials
- Waters Sep-Pak™ C18 Cartridges, Part #51910
- 200 proof ethanol
- 0.9% Sodium Chloride Injection, USP
- 0.001N hydrochloric acid (May be prepared by diluting 1 mL of 0.1N hydrochloric acid to 100 mL with Water for Injection, USP, or by other dilution of more concentrated hydrochloric acid. For example, 0.1 mL of 36% (~11.6N) hydrochloric acid diluted to a total volume of 1,150 mL)
- 1:1 ethanol: 0.9% Sodium Chloride Injection, USP (Prepared by mixing equal volumes of 200 proof ethanol and 0.9% Sodium Chloride Injection, USP)
- 10 mL disposable syringe, no needle required
- 1 mL disposable syringe, with needle
- Disposable culture tubes or vials, minimum 15 mL capacity
- Ion chamber for measurement of radioactive samples
Preparation of Sep-Pak Cartridge
- Using a 10 mL syringe, push 10 mL of 200 proof ethanol through the Sep-Pak cartridge. Discard the eluate.
- Flush the cartridge with 10 mL of the 0.001N hydrochloric acid. Discard the eluate.
- Drain the cartridge by pushing 5 mL of air through the cartridge with the syringe. Discard the eluate.
Sample Analysis
- Apply 0.1 mL of the Technetium Tc 99m Mertiatide Injection preparation to the head of the cartridge through the longer end of the cartridge using a 1 mL syringe with needle.
Note: The cartridge and all solutions eluted from it will be radioactive after this step, therefore, use lead dispensing shield to minimize radiation exposure. - With a disposable 10 mL syringe, slowly push 10 mL of 0.001N hydrochloric acid through the cartridge. Collect this fraction in a first culture tube or vial for counting.
- Elute the cartridge with 10 mL of the 1:1 ethanol: 0.9% Sodium Chloride Injection USP solution. Ensure that this solution is pushed through the cartridge slowly so that the elution occurs in a drop-wise manner. Collect this 10 mL fraction in a second culture tube or vial for counting.
- Place the Sep-Pak cartridge in a third culture tube or vial for counting.
Counting
- Assay the activity of the first fraction (in 0.001N hydrochloric acid) in an ion chamber. This fraction contains the hydrophilic impurities (free pertechnetate, technetium tartrate, etc.) and a fraction of reduced-hydrolyzed technetium.
- Assay the activity of the second fraction (in 1:1 ethanol: 0.9% Sodium Chloride Injection, USP). This fraction contains Technetium Tc 99m Mertiatide.
- Assay the activity of the Sep-Pak cartridge in the third culture tube or vial. This component contains the remaining reduced-hydrolyzed technetium and non-elutable impurities.
Calculations
1. Percent Technetium Tc 99m Mertiatide =
Activity of 2nd (1:1 ethanol: 0.9% Sodium Chloride Injection) fraction x 100%
Total activity of all 3 fractionsNote: Not less than 90% of the activity must be Technetium Tc 99m Mertiatide.
2. Percent hydrophilic impurities =
Activity of 1st (0.001N hydrochloric acid) fraction x 100%
Total activity of all 3 fractions3. Percent non-elutable impurities =
Activity remaining on Sep-Pak cartridge x 100%
Total activity of all 3 fractionsNote: Total impurities must not be more than 10%.
2.6 Administration Instructions
- Measure the patient dose using a suitable radioactivity calibration system immediately prior to administration.
- Visually inspect the prepared Technetium Tc 99m Mertiatide Injection behind a lead glass shield for particulate matter and discoloration prior to administration. Only use solutions that are clear without visible particles.
- Use aseptic procedures and a shielded syringe to withdraw doses for administration to patients. Wear waterproof gloves during the administration procedure.
2.7 Radiation Dosimetry
The estimated radiation doses per injected activity of Technetium Tc 99m Mertiatide Injection by an intravenous administration are presented in Table 1.
Table 1. Estimated Radiation Equivalent Dose for Intravenously Administered Technetium Tc 99m Mertiatide*
Radiation Equivalent Dose per Unit Activity Administered (mSv/MBq) Organ Adult 15 Years 10 Years 5 Years 1 Year 8 Days** Gallbladder Wall 0.0044 0.0053 0.007 0.011 0.034 0.073 Lower Large Intestine Wall 0.0088 0.011 0.01 0.016 0.022 0.047 Small Intestine 0.0044 0.0055 0.0059 0.0085 0.0075 0.014 Upper Large Intestine Wall 0.0051 0.0066 0.0086 0.013 0.013 0.026 Kidneys 0.0039 0.0047 0.0064 0.0093 0.015 0.038 Liver 0.00098 0.0013 0.0018 0.0028 0.0042 0.0087 Ovaries 0.007 0.009 0.0065 0.0094 0.0085 0.016 Red Marrow 0.0013 0.0017 0.0015 0.002 0.0022 0.0044 Testes 0.0044 0.0064 0.005 0.0077 0.0073 0.014 Urinary Bladder Wall 0.13 0.16 0.1 0.15 0.13 0.31 Total Body 0.0018 0.0022 0.0017 0.0026 0.003 0.0045 *Calculated by Oak Ridge Associated Universities, based upon the pediatric phantom series of Christy and Eckerman of Oak Ridge National Laboratories. The adult radiation absorbed doses were calculated based on data from 10 healthy subjects using the Medical Internal Radiation Dose Committee (MIRD) schema.
** Technetium Tc 99m Mertiatide Injection is not approved for pediatric patients younger than 30 days old [see Indications and Usage (1)]. -
3 DOSAGE FORMS AND STRENGTHS
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection contains 1 mg betiatide supplied as a white lyophilized powder packaged in 10 mL glass multiple-dose vials. Radiolabeling with sodium pertechnetate Tc 99m injection provides a clear, colorless solution containing up to 3,700 MBq (100 mCi) technetium Tc 99m mertiatide in approximately 10 mL volume at calibration time..
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
Technetium Tc 99m Mertiatide Injection contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Ensure safe handling to minimize radiation exposure to the patient and healthcare providers. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.2)].
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of Technetium Tc 99m Mertiatide Injection were identified in clinical studies or postmarketing reports. Because some of these reactions are voluntarily reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: tachycardia.
Gastrointestinal disorders: nausea, vomiting.
General disorders and administration site conditions: shaking chills, fever.
Nervous system disorders: seizure.
Respiratory, thoracic and mediastinal disorders: wheezing, dyspnea.
Skin and subcutaneous tissue disorders: itching, rash.
Vascular disorders: elevation in blood pressure. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with technetium Tc 99m mertiatide use in pregnant women to determine a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. No animal reproductive studies have been conducted with technetium Tc 99m mertiatide. Although all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose, the radiation exposure to the fetus from technetium Tc 99m mertiatide is expected to be low (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Data
Human Data
No adverse fetal effects of radiation risks have been identified for diagnostic procedures involving less than 50 mGy, which represents less than 10 mGy fetal doses.
8.2 Lactation
Risk Summary
There are no data on the presence of technetium Tc 99m mertiatide in breast milk, however, published case reports describe the presence of technetium Tc 99m in breast milk. There are no data on the effects of technetium Tc 99m mertiatide on the breastfed infant or the effects on milk production. Based on clinical guidelines, exposure of technetium Tc 99m mertiatide to a breastfed infant may be minimized by advising a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for a minimum of 24 hours after administration of Technetium Tc 99m Mertiatide Injection. The developmental and health benefits of breastfeeding should be considered along with a mother’s clinical need for Technetium Tc 99m Mertiatide Injection and any potential adverse effects on the breastfed child from technetium Tc 99m mertiatide or from the underlying maternal condition.
8.4 Pediatric Use
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection, after radiolabeling with technetium-99m, is indicated for use in the diagnosis of congenital and acquired renal abnormalities, renal failure, urinary tract obstruction, and calculi in pediatric patients aged 30 days and older.
The safety and effectiveness of Kit for the Preparation of Technetium Tc 99m Mertiatide Injection have not been established in pediatric patients younger than 30 days old.
- 10 OVERDOSAGE
-
11 DESCRIPTION
11.1 Chemical Characteristics
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection is a sterile, multiple-dose kit for the preparation of technetium Tc 99m mertiatide injection, a radioactive diagnostic agent for intravenous use.
The active ingredient, betiatide, is N-[N-[N-[(benzoylthio) acetyl]glycyl]glycyl]-glycine and has a molecular weight of 367.38 g/mol. After reconstitution with sodium pertechnetate Tc 99m injection, the radioactive agent technetium Tc 99m mertiatide (disodium[N-[N-[N- (mercaptoacetyl) glycyl]glycyl] glycinato (2-) - N,N′,N″,S′]oxotechnetate (2-)) is formed in situ.
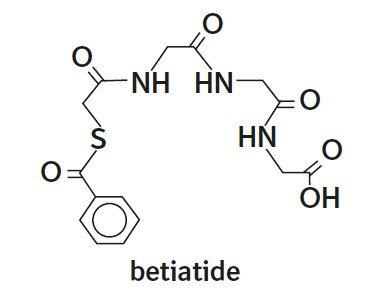
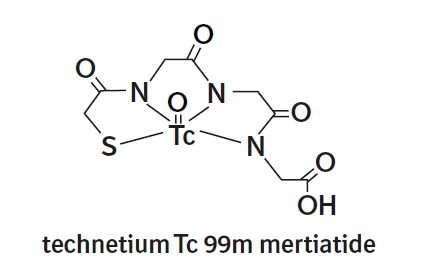
Each 10 mL vial contains 1 mg betiatide, 20 mg lactose monohydrate, 40 mg sodium tartrate dihydrate, 0.07 mg (minimum) stannous chloride dihydrate and 0.2 mg (maximum) total tin expressed as stannous chloride dihydrate. Prior to lyophilization, hydrochloric acid is added for pH adjustment. The vial contents are sealed under argon.
After radiolabeling with sodium pertechnetate Tc 99m injection, each vial contains up to 3,700 MBq (100 mCi) of technetium Tc 99m mertiatide in 0.9% sodium chloride injection in approximately 10 mL volume as a sterile, clear, and colorless solution with a pH between 5 and 6.
11.2 Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging is listed in Table 2.
Table 2. Principal Radiation Emission Data Radiation M Mean % per Disintegration Energy (keV) Gamma-2 89.07 140.5 To correct for physical decay of the radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 3.
Table 3. Physical Decay Chart: Technetium Tc 99m, Half-life 6.02 Hours Hours Fraction Remaining Hours Fraction Remaining 0* 1.000 7 0.447 1 0.891 8 0.398 2 0.794 9 0.355 3 0.708 10 0.316 4 0.631 11 0.282 5 0.562 12 0.251 6 0.501 * Calibration Time
11.3 External Radiation
The specific gamma ray constant for technetium-99m is 0.78 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for technetium-99m is 0.017 cm. The use of 0.25 cm of Pb, for example, will decrease the external radiation exposure by a factor of about 1,000. Table 4 displays the radiation attenuation by lead shielding.
Table 4. Radiation Attenuation by Lead Shielding Shield Thickness (Pb) cm Coefficient of Attenuation 0.017 0.5 0.08 10-1 0.16 10-2 0.25 10-3 0.33 10-4 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Following intravenous administration for renal imaging, Technetium Tc 99m Mertiatide is rapidly cleared from the blood. It is cleared by the kidneys and excreted in the urine.
12.2 Pharmacodynamics
Following intravenous injection of technetium Tc 99m mertiatide, the uptake, concentration, and excretion of the tracer by the kidney can be monitored to assess renal function.
12.3 Pharmacokinetics
Distribution
After an intravenous administration in healthy adult subjects, 89% of technetium Tc 99m mertiatide was plasma-protein bound. The protein binding is reversible.
Elimination
Excretion
In healthy subjects, the plasma clearance was approximately 0.3 liters/minute and the amount of technetium Tc 99m mertiatide excreted in the urine in 3 hours was nearly 90% of the injected dose.
Specific Populations
Patients with Renal Impairment
In a study performed in three patients with renal impairment (serum creatinine greater than 6.3 mg/dL), 78% of the tracer was plasma protein bound after intravenous injection. The mean plasma clearance of technetium Tc 99m mertiatide was 0.03 liters/minute and 21.3% was excreted in 3 hours on average. In both healthy subjects and patients with renal impairment, the plasma concentration-time profile showed a biexponential decline.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection containing 1 mg betiatide, is supplied as a white lyophilized powder packaged in a sterile, multiple-dose 10 mL vial as a carton of 5 vials (NDC 65174-261-05). The carton also contains:
- 5 venting needles with filter.
- 5 radioassay information labels for the radiolabeled product.
The radionuclide is not part of Kit for the Preparation of Technetium Tc 99m Mertiatide Injection.
Storage and Handling
Before radiolabeling, store at controlled room temperature 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature] and protect from light.
After radiolabeling with sodium pertechnetate Tc 99m injection, store vial upright in shielding to protect from radiation at room temperature 15°C to 30°C (59°F to 86°F). Use within 6 hours of radiolabeling.
Dispose of unused Technetium Tc 99m Mertiatide Injection in compliance with the regulations of the government agency authorized to license the use of this radionuclide.
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
-
17 PATIENT COUNSELING INFORMATION
Adequate Hydration
Advise patients to drink sufficient amount of water to ensure adequate hydration before administration of Technetium Tc 99m Mertiatide Injection and to continue to drink and void frequently following administration to minimize radiation dose [see Dosage and Administration (2.2) and Warning and Precautions (5.1)].
Pregnancy:
Advise pregnant women of the risk of fetal exposure to radiation doses if they undergo a radionuclide procedure [see Use in Specific Populations (8.1)].
Lactation:
Advise a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for at least 24 hours after Technetium Tc 99m Mertiatide Injection administration to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].Manufactured for:
Jubilant DraxImage Inc., dba Jubilant Radiopharma™
16 751 TransCanada Highway
Kirkland, Quebec H9H 4J4 Canada
1-888-633-5343
www.jubilantradiopharma.com
Art Rev.: 3.0Sep-Pak™ is a trademark of Waters Technologies Corporation.
Jubilant Radiopharma™ is a trademark used under license by Jubilant DraxImage Inc. -
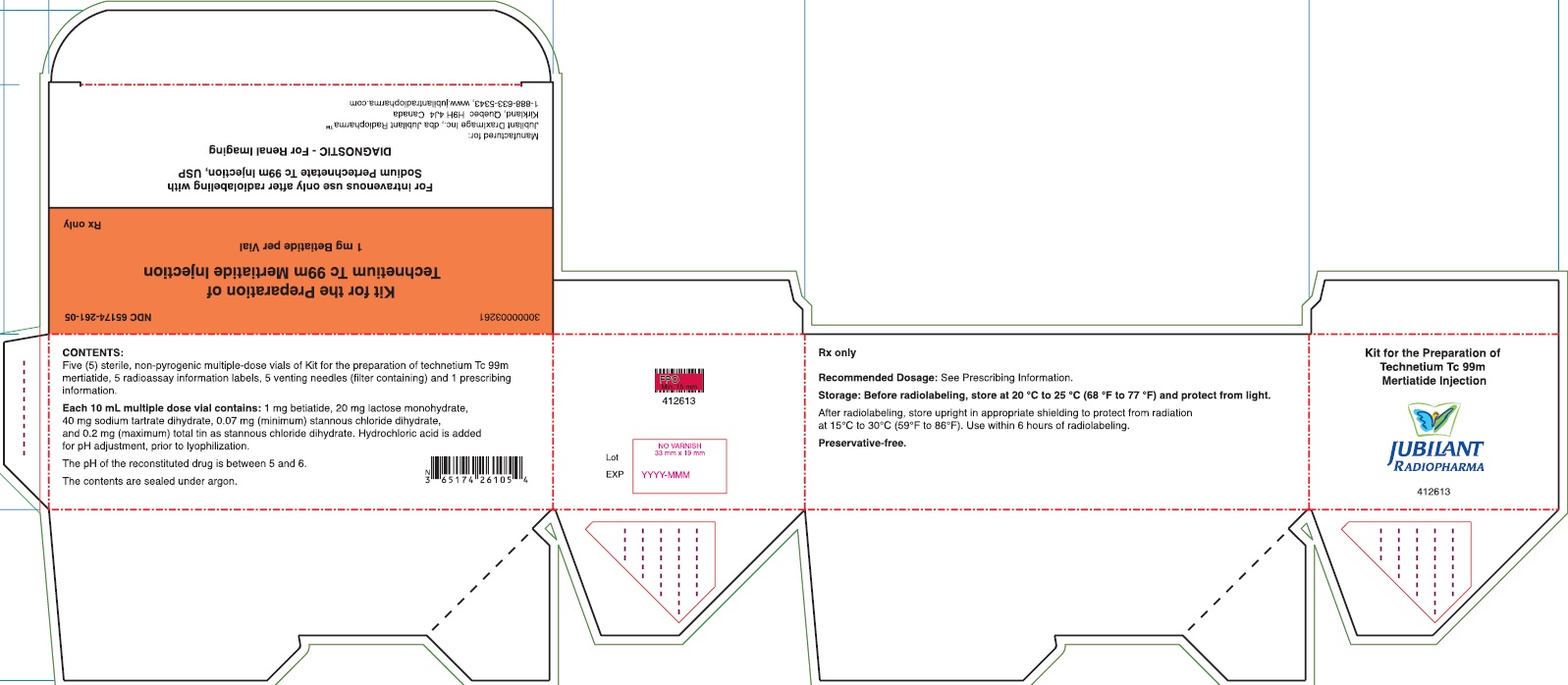
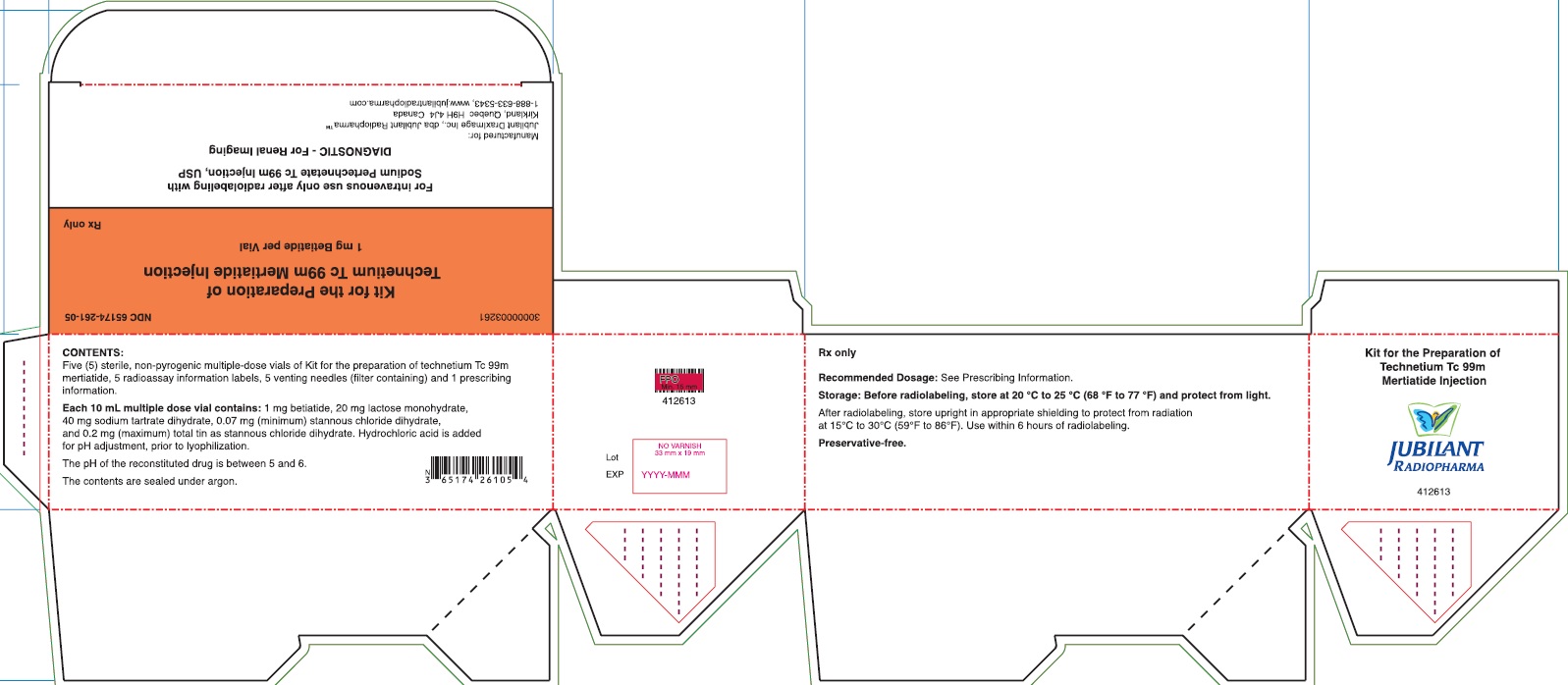
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 5 VIAL BOX
NDC 65174-261-05
Kit for the Preparation of Technetium Tc 99m Mertiatide
1 mg Betiatide per Vial
Rx onlyFor intravenous use only after radiolabeling with
Sodium Pertechnetate Tc 99m Injection, USP
DIAGNOSTIC - For Renal Imaging
Manufactured for:
Jubilant DraxImage Inc., dba Jubilant Radiopharma™
Kirkland, Quebec H9H 4J4 Canada
1-888-633-5343, www.jubilantradiopharma.comCONTENTS:
Five (5) sterile, non-pyrogenic multiple-dose vials of Kit for the preparation of technetium Tc 99m mertiatide, 5 radioassay information labels, 5 venting needles (filter containing) and 1 prescribing information.Each 10 mL multiple dose vial contains: 1 mg betiatide, 20 mg lactose monohydrate, 40 mg tartrate dihydrate, 0.07 (minimum) stannous chloride dihydrate, and 0.2 mg (maximum) total tin as stannous chloride dihydrate. Hydrochloric acid is added for pH adjustment, prior to lyophilization.
The pH of the reconstituted drug is between 5 and 6.
The contents are sealed under argon.
Rx only
Recommended Dosage: See Prescrbing Information.
Storage: Before radiolabeling, store at 20 °C to 25 °C (68 °F to 77 °F) and protect from light.
After radiolabeling, store upright inappropriate shielding to protect from radiation at 15°C to 30°C (59°F to 86°F). Use within 6 hours of radiolabeling.
Preservative-free.
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - VIAL CONTAINER (10 mL Multi-Dose Reaction Vial)
Sterile NDC 65174-261-01
Kit for the Preparation of Technetium Tc 99m Mertiatide Injection
1 mg Betiatide per Vial Rx onlyMultiple-dose reaction vial
For intravenous use only after radiolabeling with Sodium Pertechnetate Tc 99m Injection USP.
Recommended Dosage: See Prescribing information.
Store at 20 °C to 25 °C (68 °F to 77 °F) and protect from light. Use within 6 hours of radiolabeling.
Manufactured for:
Jubilant DraxImage Inc.
Kirkland, Quebec, Canada H9H 4J4
-
INGREDIENTS AND APPEARANCE
TECHNETIUM TC 99M MERTIATIDE
technetium tc 99m mertiatide injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65174-261 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETIATIDE (UNII: 9NV2SR34P8) (BETIATIDE - UNII:9NV2SR34P8) BETIATIDE 1 mg Inactive Ingredients Ingredient Name Strength STANNOUS CHLORIDE (UNII: 1BQV3749L5) 0.2 mg SODIUM TARTRATE DIHYDRATE (UNII: DIA7C37AOW) 40 mg LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) 20 mg HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65174-261-05 5 in 1 CARTON 03/31/2023 1 NDC:65174-261-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216820 03/31/2023 Labeler - Jubilant DraxImage Inc., dba Jubilant Radiopharma (243604761) Registrant - Jubilant DraxImage Inc., dba Jubilant Radiopharma (243604761) Establishment Name Address ID/FEI Business Operations Jubilant DraxImage Inc., dba Jubilant Radiopharma 243604761 analysis(65174-261) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier General Partnership 246762764 manufacture(65174-261)