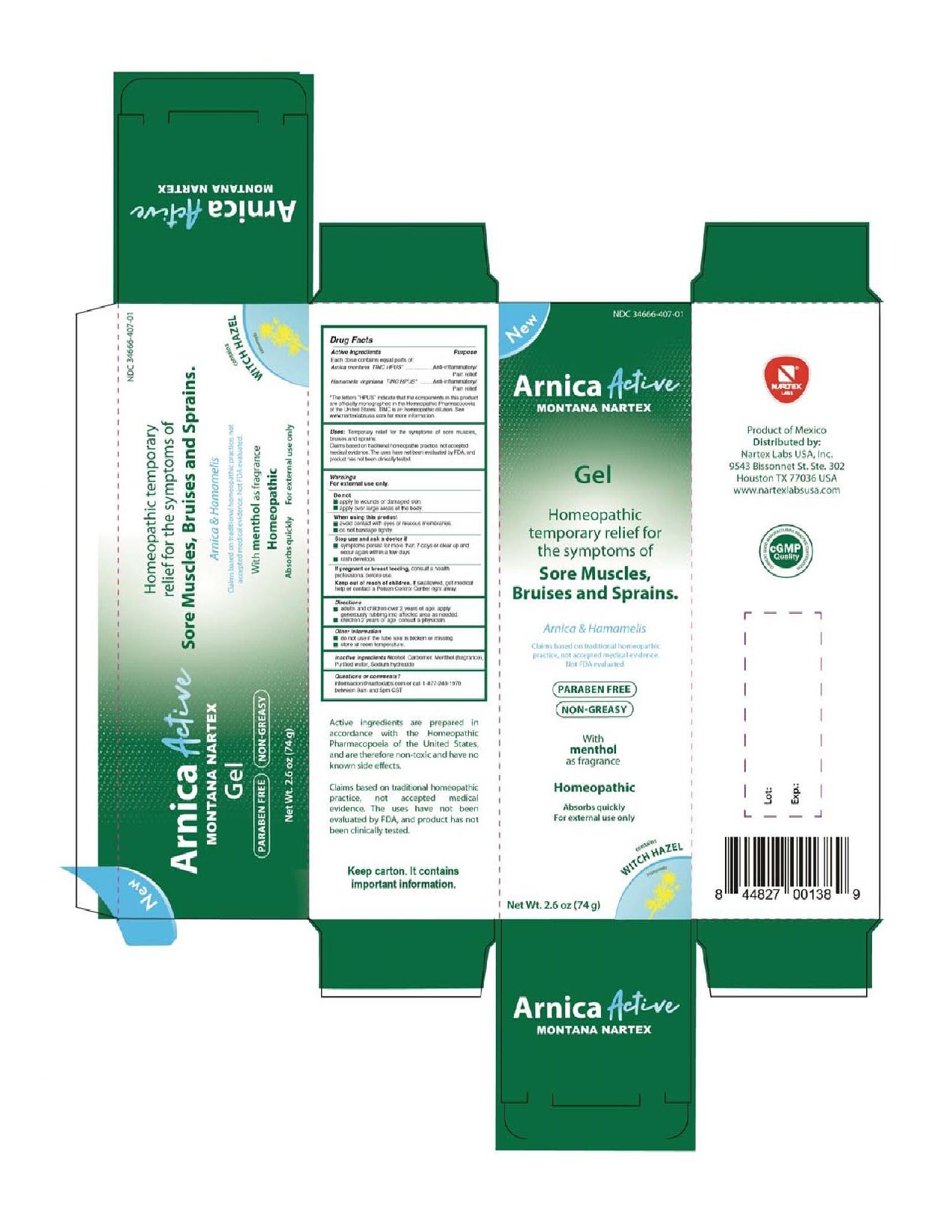
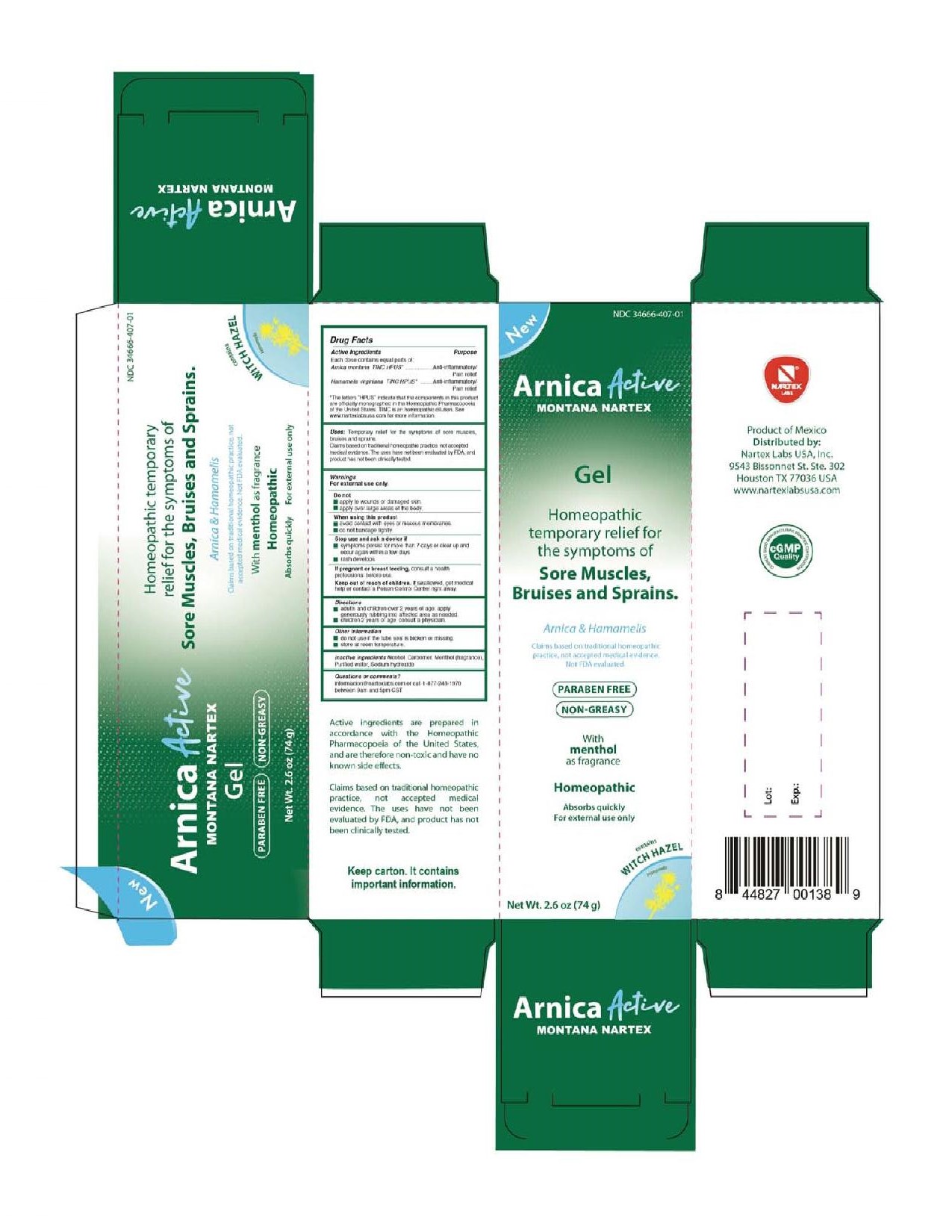
Label: ARNICA ACTIVE MONTANA NARTEX- arnica montana, hamamelis virginiana gel
- NDC Code(s): 34666-407-01
- Packager: Nartex Laboratorios Homeopaticos, S.A. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients
Each dose contains equal parts of:
Arnica montana TINC HPUS*
Hamamelis virginia TINC HPUS*
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. TINC is an homeopathic dilution. See www.nartexlabsusa.com for more information.
-
Purpose
Each dose contains equal parts of:
Arnica montana TINC HPUS*..................Anti-inflammatory/Pain relief
Hamamelis virginia TINC HPUS*.........................Anti-inflammatory/Pain relief
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. TINC is an homeopathic dilution. See www.nartexlabsusa.com for more information.
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
New
NDC 34666-407-01
Arnica Active Montana Nartex
Gel
Homeopathic temporary relief for the symptoms of Sore Muscles, Bruises and Sprains.
Arnica & Hamamelis
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Paraben Free
Non-Greasy
With menthol as fragrance
Homeopathic
Absorbs quickly
For external use only
contains Witch Hazel
Hamamelis
Net Wt. 2.6 oz (74 g)

-
INGREDIENTS AND APPEARANCE
ARNICA ACTIVE MONTANA NARTEX
arnica montana, hamamelis virginiana gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34666-407 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 74 g HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 1 [hp_X] in 74 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) MENTHOL (UNII: L7T10EIP3A) SODIUM HYDROXIDE (UNII: 55X04QC32I) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34666-407-01 1 in 1 CARTON 12/30/2021 1 74 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/30/2021 Labeler - Nartex Laboratorios Homeopaticos, S.A. de C.V. (589914576) Establishment Name Address ID/FEI Business Operations Nartex Laboratorios Homeopaticos, S.A. de C.V. 589914576 manufacture(34666-407)