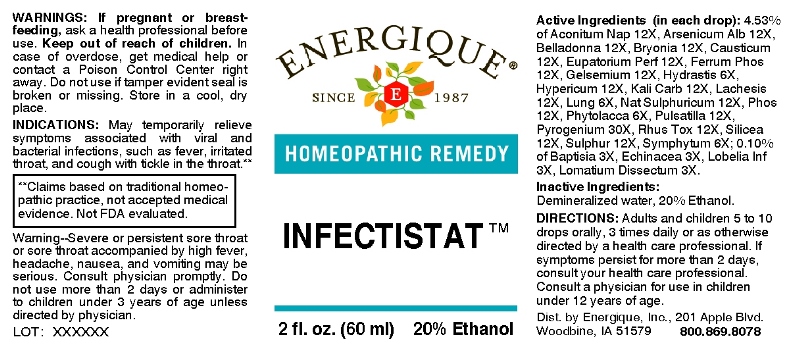
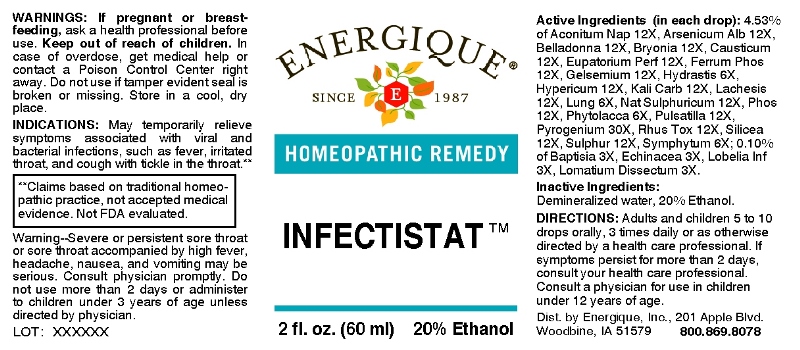
Label: INFECTISTAT (baptisia tinctoria, echinacea (angustifolia), lobelia inflata, lomatium dissectum, hydrastis canadensis, lung (suis), phytolacca decandra, symphytum officinale, aconitum napellus, arsenicum album, belladonna, bryonia (alba), causticum, eupatorium perfoliatum, ferrum phosphoricum, gelsemium sempervirens, hypericum perforatum, kali carbonicum, lachesis mutus, natrum sulphuricum, phosphorus, pulsatilla- pratensis, rhus tox, silicea, sulphur, pyrogenium liquid
- NDC Code(s): 44911-0504-1
- Packager: Energique, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 22, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
(in each drop): 4.53% of Aconitum Napellus 12X, Arsenicum Album 12X, Belladonna 12X, Bryonia 12X, Causticum 12X, Eupatorium Perfoliatum 12X, Ferrum Phosphoricum 12X, Gelsemium Sempervirens 12X, Hydrastis Canadensis 6X, Hypericum Perforatum 12X, Kali Carbonicum 12X, Lachesis Mutus 12X, Lung (Suis) 6X, Natrum Sulphuricum 12X, Phosphorus 12X, Phytolacca Decandra 4X, Pulsatilla (Pratensis) 12X, Pyrogenium 30X, Rhus Tox 12X, Silicea 12X, Sulphur 12X, Symphytum Officinale 6X,; 0.10% of Baptisia Tinctoria 3X, Echinacea (Angustifolia) 3X, Lobelia Inflata 3X, Lomatium Dissectum 3X.
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist for more than 2 days, consult your health care professional. Consult a physician for use in children under 12 years of age.
WARNING: Severe or persistent sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a physician promptly. Do not use more than two days or administer to children under 3 years of age unless directed by a physician.
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
INFECTISTAT
baptisia tinctoria, echinacea (angustifolia), lobelia inflata, lomatium dissectum, hydrastis canadensis, lung (suis), phytolacca decandra, symphytum officinale, aconitum napellus, arsenicum album, belladonna, bryonia (alba), causticum, eupatorium perfoliatum, ferrum phosphoricum, gelsemium sempervirens, hypericum perforatum, kali carbonicum, lachesis mutus, natrum sulphuricum, phosphorus, pulsatilla (pratensis), rhus tox, silicea, sulphur, pyrogenium liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 1 mL LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 3 [hp_X] in 1 mL LOMATIUM DISSECTUM ROOT (UNII: 5329928G5N) (LOMATIUM DISSECTUM ROOT - UNII:5329928G5N) LOMATIUM DISSECTUM ROOT 3 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 6 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL SUS SCROFA LUNG (UNII: 7GL3G1COB3) (SUS SCROFA LUNG - UNII:7GL3G1COB3) SUS SCROFA LUNG 6 [hp_X] in 1 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 1 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 12 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 12 [hp_X] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] in 1 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 12 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] in 1 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 12 [hp_X] in 1 mL POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 12 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 12 [hp_X] in 1 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL ANEMONE PRATENSIS (UNII: 8E272251DI) (ANEMONE PRATENSIS - UNII:8E272251DI) ANEMONE PRATENSIS 12 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 30 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0504-1 60 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 08/01/2019 10/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/2019 10/24/2024 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0504) , api manufacture(44911-0504) , label(44911-0504) , pack(44911-0504)