Label: HEMPVANA PAIN RELIEF- trolamine salicylate cream
- NDC Code(s): 39765-011-01
- Packager: Neutraderm, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin
- Do not use at the same time as other topical analgesics.
Stop use and ask a doctor if
- Condition worsens
- Symptoms persist for more than 7 days or clear up and occur again within a few days
- Redness is present or irritation develops
If pregnant or breast-feeding, ask a health professional before use.
- Directions
-
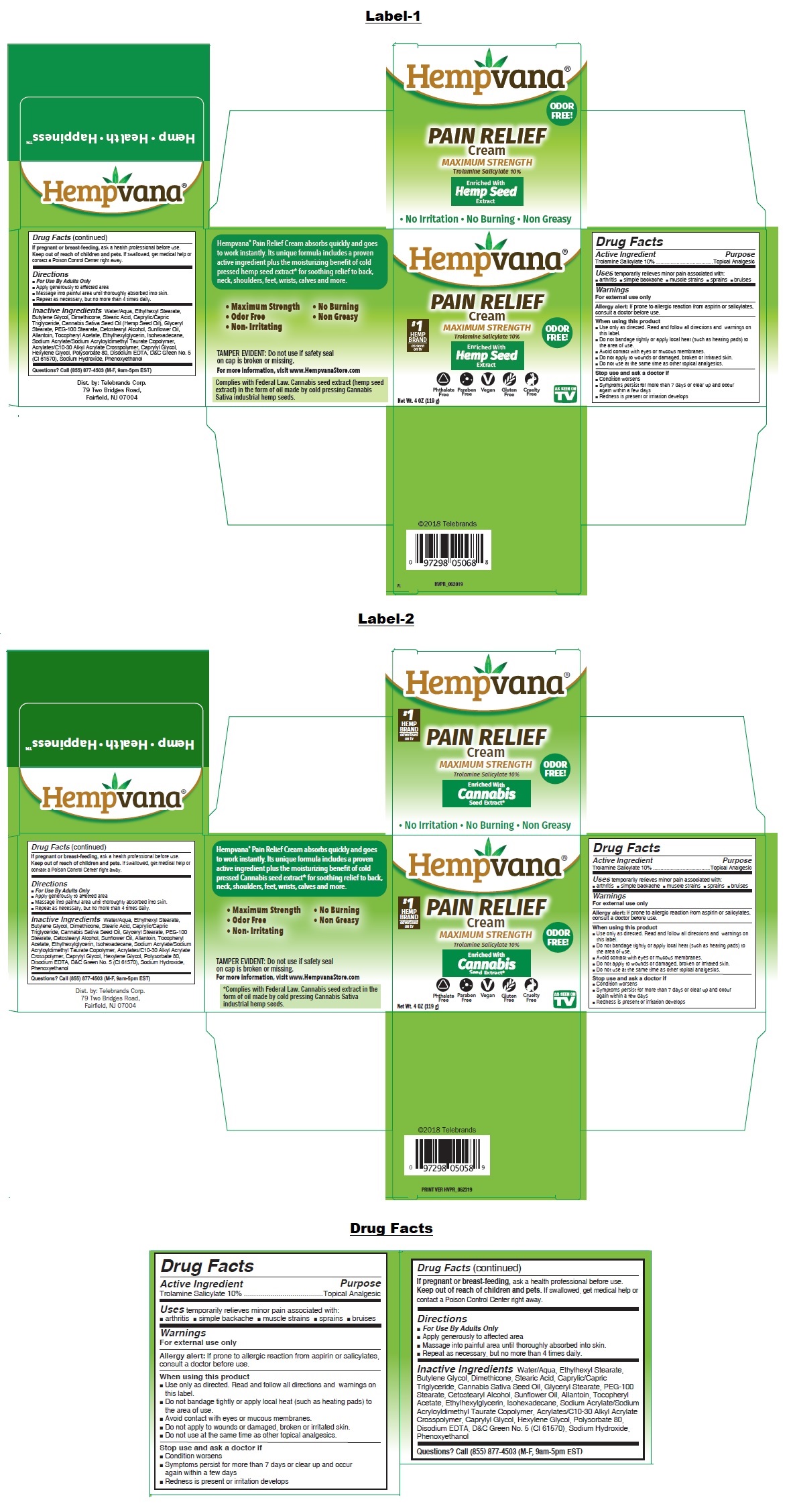
Inactive Ingredients
Water/Aqua, Ethylhexyl Stearate, Butylene Glycol, Dimethicone, Stearic Acid, Caprylic/Capric Triglyceride, Cannabis Sativa Seed Oil (Hemp Seed Oil), Glyceryl Stearate, PEG-100 Stearate, Cetostearyl Alcohol, Sunflower Oil, Allantoin, Tocopheryl Acetate, Ethylhexylglycerin, Isohexadecane,
Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Hexylene Glycol, Polysorbate 80, Disodium EDTA, D&C Green No. 5 (CI 61570), Sodium Hydroxide, Phenoxyethanol - Questions?
-
SPL UNCLASSIFIED SECTION
MAXIMUM STRENGTH
Enriched With Hemp Seed* Extract
ODOR FREE!
Phthalate Free Paraben Free Vegan Gluten Free Cruelty Free
• No Irritation • No Burning • Non Greasy
Hempvana® Pain Relief Cream absorbs quickly and goes to work instantly. Its unique formula includes a proven active ingredient plus the moisturizing benefit of cold pressed hemp seed* extract for soothing relief to back, neck, shoulders, feet, wrists, calves and more.
TAMPER EVIDENT: Do not use if safety seal on cap is broken or missing.
For more information, visit www.HempvanaStore.com
*Complies with Federal Law. Hemp seed extract contains no detectible tetrahydrocannabinol (THC) or cannabidiol (CBD), Derived from the cannabis industrial hemp cultivar.
Dist. by: Telebrands Corp.
79 Two Bridges Road,
Fairfield, NJ 07004
Hemp • Health • HappinessTM
- Packaging
-
INGREDIENTS AND APPEARANCE
HEMPVANA PAIN RELIEF
trolamine salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39765-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROLAMINE SALICYLATE (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) TROLAMINE SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) STEARIC ACID (UNII: 4ELV7Z65AP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SUNFLOWER OIL (UNII: 3W1JG795YI) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOHEXADECANE (UNII: 918X1OUF1E) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) Product Characteristics Color green (Pale Green to Light Green) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39765-011-01 1 in 1 CARTON 01/10/2019 1 119 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/10/2019 Labeler - Neutraderm, Inc. (146224444) Establishment Name Address ID/FEI Business Operations Neutraderm, Inc. 146224444 manufacture(39765-011)