Label: SLEEP-AID, LIL DRUG STORE- diphenhydramine hcl caplet, 25mg tablet, film coated
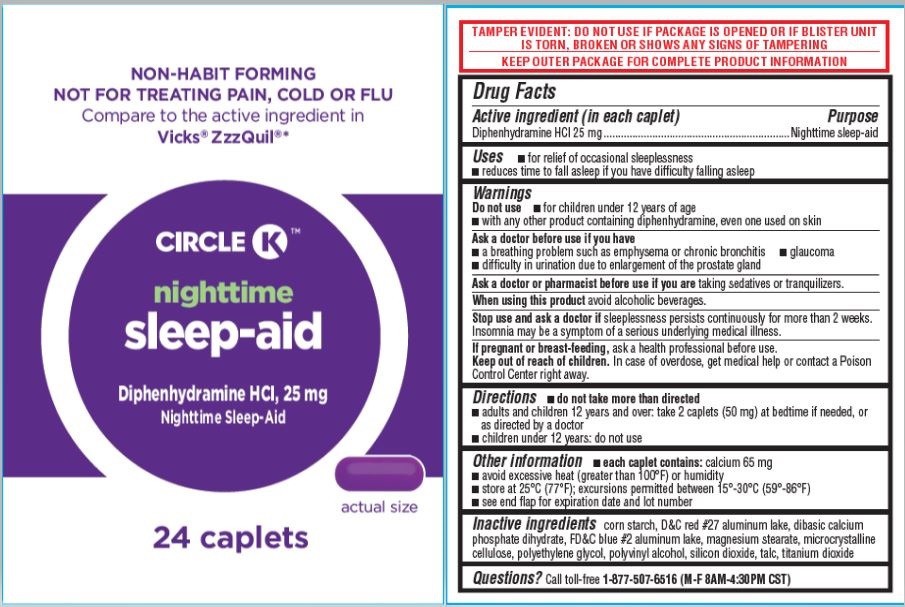
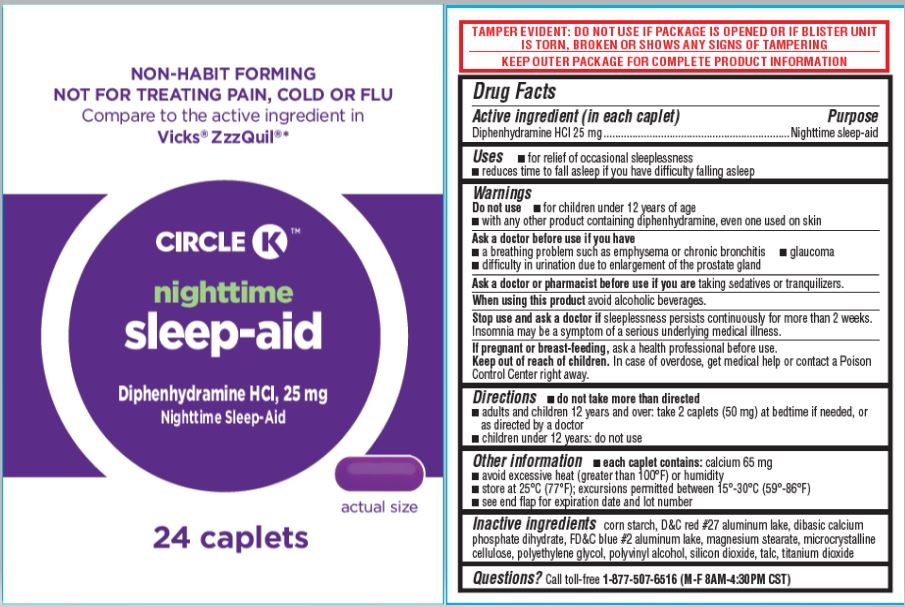
SLEEP-AID, CIRCLE K- diphenhydramine hcl caplet, 25mg tablet, film coated
- NDC Code(s): 66715-5503-8, 66715-6643-8
- Packager: Lil' Drug Store Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions?
- PDP [Lil' Drug Store Nighttime Sleep-Aid, 24ct]
- PDP [Circle K Nighttime Sleep-Aid, 24ct]
-
INGREDIENTS AND APPEARANCE
SLEEP-AID, LIL DRUG STORE
diphenhydramine hcl caplet, 25mg tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-6643 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color purple Score no score Shape OVAL (Biconvex capsule-shaped) Size 14mm Flavor Imprint Code 44;672 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-6643-8 2 in 1 CARTON 12/20/2021 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 12/20/2021 SLEEP-AID, CIRCLE K
diphenhydramine hcl caplet, 25mg tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-5503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color purple Score no score Shape OVAL (Biconvex capsule-shaped) Size 14mm Flavor Imprint Code 44;672 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-5503-8 2 in 1 CARTON 12/20/2021 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 12/20/2021 Labeler - Lil' Drug Store Products, Inc. (093103646)