Label: AQUAPHOR ORIGINAL- petrolatum ointment
- NDC Code(s): 10356-100-37
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
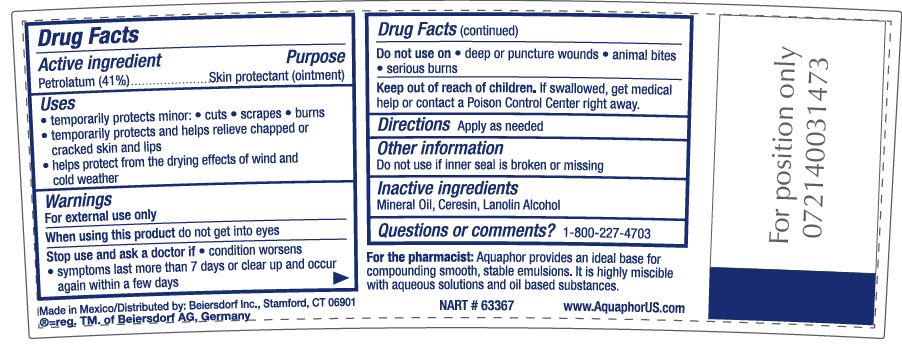
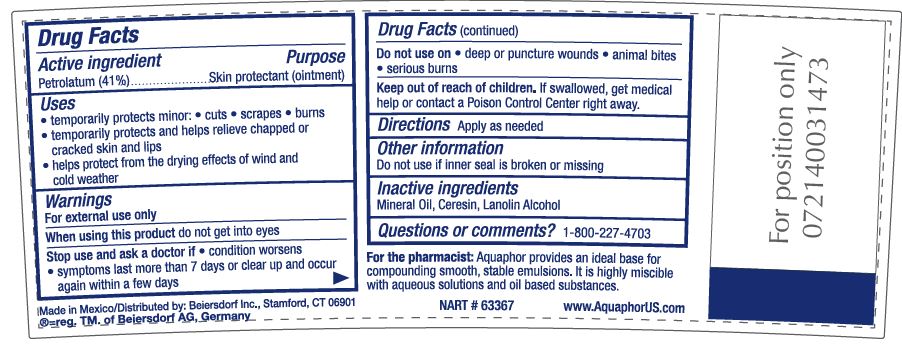
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- QUESTIONS
- DOSAGE & ADMINISTRATION
-
PRINCIPAL DISPLAY PANEL

Aquaphor Original Ointment
Severly Dry Skin Treatment
Clinically proven to restore smooth, healthy skin
Preservative and Fragrance Free
Skin Protectant
Dermatologist recommended
For the pharmacist: Aquaphor provides an ideal base for compounding smooth, stable emulsions.
It is highly miscible with aqueous solutions as well as oil-based substances.
-
INGREDIENTS AND APPEARANCE
AQUAPHOR ORIGINAL
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 41 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) CERESIN (UNII: Q1LS2UJO3A) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-100-37 396 g in 1 JAR; Type 0: Not a Combination Product 06/01/1975 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/01/1975 Labeler - Beiersdorf Inc (001177906)