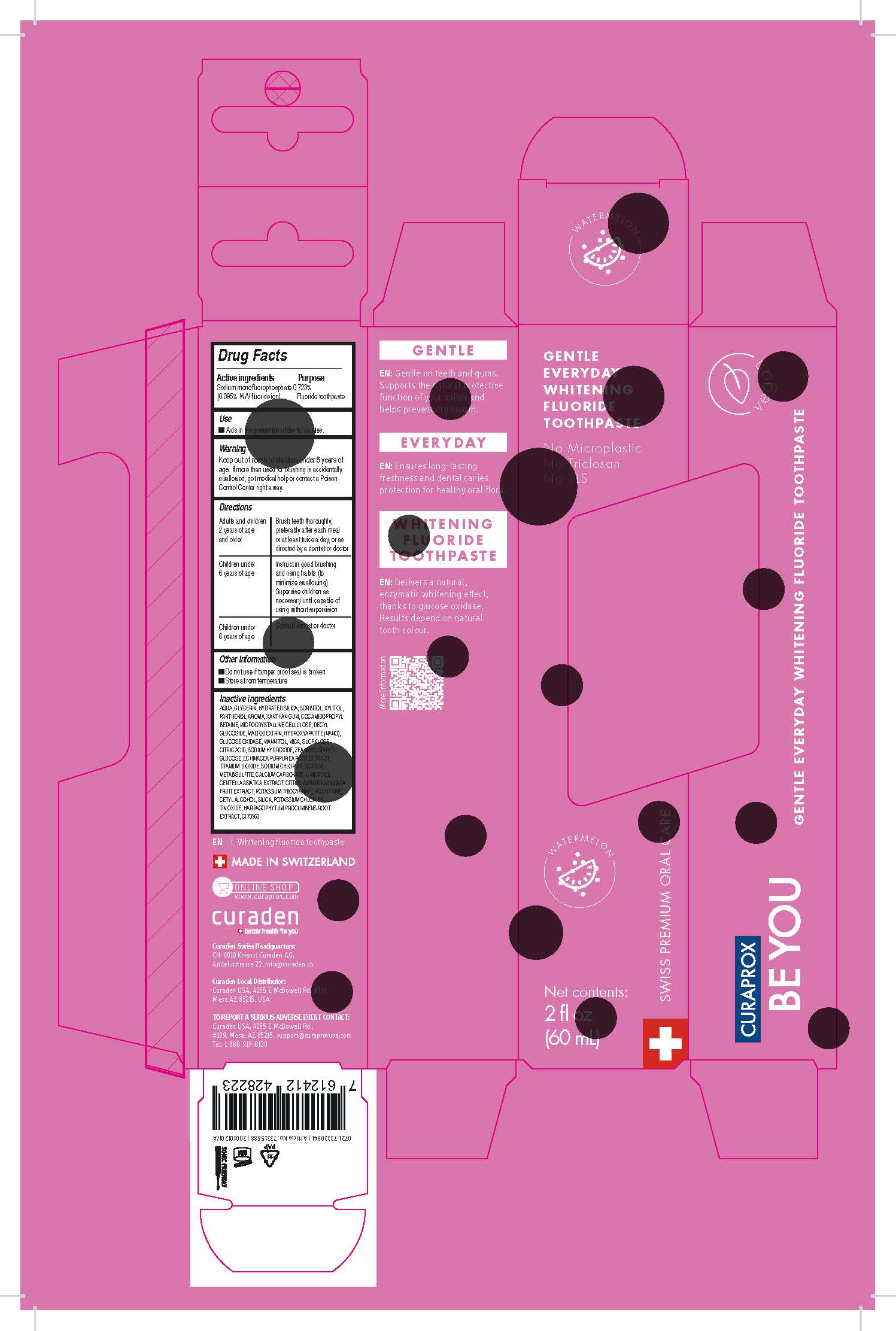
Label: BEYOU PINK- toothpaste paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 71112-002-20 - Packager: Curaden AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Specific Warning for OTC
-
Directions
Adults and children 2 years of age and older: Brush teeth thoroughly, preferably afler each meal or at least twice a day, or as directed by a dentist or doctor
Children under 6 years of age: Instructin good brushing and rising habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision
Children under 6 years of age: Consult dentist or doctor
- Other information
-
Inactive ingredients
AQUA, GLYCERIN, HYDRATED SILICA, SORBITOL, XYLITOL, PANTHENOL, AROMA, XANTHAN GUM, COCAMIDOPROPYL BETAINE, MICRCX::RYSTALLINE CELlULOSE, DECYL GLUCOSIDE, MALTODEXTRIN, HYDROXYAPATITE (NANO), GLUCOSE OXIDASE, MANNITOL, MICA, SUCRALOSE, CITRIC ACID, SODIUM HYDROXIDE, ZEA MAYS STARCH, GLUCOSE, ECHINACEA PURPUREA ROOT EXTRACT, TITANIUM DIOXIDE, SODIUM CHLORIDE, SODIUM METABISULFITE, CALCIUM CARBONATE, L-MENTHOL, CENTELLA ASIATICA EXTRACT, CITRUS AURANTIUM AMARA FRUIT EXTRACT, POTASSIUM THIOCYANATE, POLYLYSINE, CETYLALCOHOL, SILICA, POTASSIUM CHLORIDE, TIN OXIDE, HARPAGOPHYTlJM PRCX::UMBENS ROOT EXTRACT, Cl 73360 (D&C Red 30)
-
Dose and Methods per Use
A pea-sized amount of toothpaste is enough. For the best whitening results: Do not rinse your mouth afterwards. Be gentle when brushing and use a toothbrush with soft, high-density bristles. Once a day, brush between your teeth with an interdental brush to keep your gums and teeth healthier, and your breath fresher.
- Label Artwork
-
INGREDIENTS AND APPEARANCE
BEYOU PINK
toothpaste paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71112-002 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.095 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM THIOCYANATE (UNII: TM7213864A) Product Characteristics Color Score Shape Size Flavor WATERMELON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71112-002-20 1 in 1 BOX 12/17/2021 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 12/17/2021 Labeler - Curaden AG (481145555) Registrant - Curaden AG (481145555) Establishment Name Address ID/FEI Business Operations Trybol AG 480305077 manufacture(71112-002)