Label: TOBCHARM- undecylenic acid liquid
- NDC Code(s): 83462-002-01
- Packager: EUbizrival LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
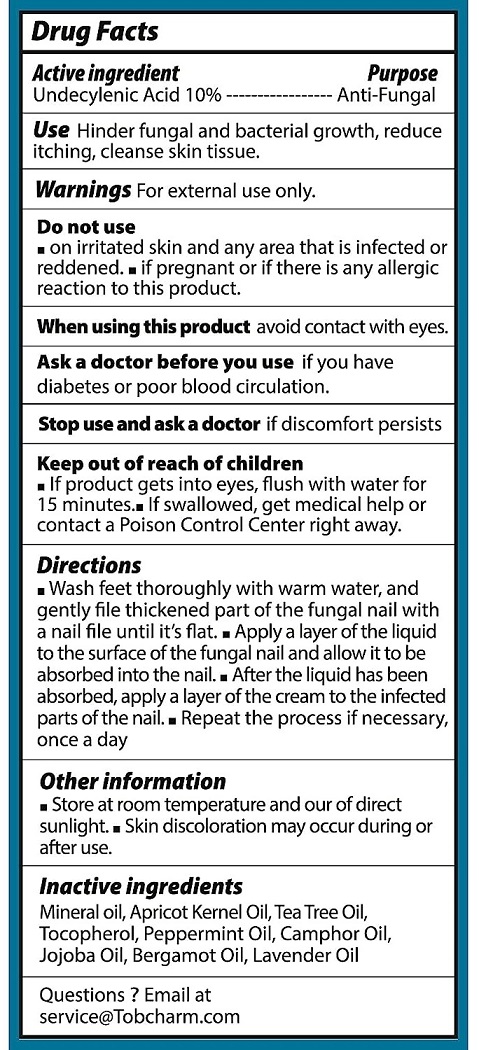
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on irritated skin and any area that is infected or reddened
- if pregnant or if there is any allergic reaction to this product.
When using this product
- avoid contact with eyes.
Stop use and ask a doctor if
- if discomfort persists
Keep out of reach of children.
- if product get into eyes, flush with water for 15 minutes
- if swallowed, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Wash feet thoroughly with warm water, and gently file thickened part of the fungal nail with a nail file until its flat
- apply layer of the liquid to the surface of the fungal nail and allow it to be absorbed into the nail.
- after the liquid has been absorbed, apply a layer of the cream to the infected parts of the nail
- repeat the process if necessary once a day
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Product label
-
INGREDIENTS AND APPEARANCE
TOBCHARM
undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83462-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 10 g in 100 mL Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) APRICOT KERNEL OIL (UNII: 54JB35T06A) TEA TREE OIL (UNII: VIF565UC2G) TOCOPHEROL (UNII: R0ZB2556P8) PEPPERMINT OIL (UNII: AV092KU4JH) CAMPHOR OIL (UNII: 75IZZ8Y727) JOJOBA OIL (UNII: 724GKU717M) BERGAMOT OIL (UNII: 39W1PKE3JI) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83462-002-01 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 09/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 09/05/2023 Labeler - EUbizrival LLC (036572203) Establishment Name Address ID/FEI Business Operations Guangzhou Pallas Cosmetics Co.,Ltd. 419564106 manufacture(83462-002)