Label: ALLANTOIN .4%- vetracare pet liquid bandage liquid
- NDC Code(s): 51098-501-01
- Packager: avadim II, LLC
- This is a repackaged label.
- Source NDC Code(s): 51098-742
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
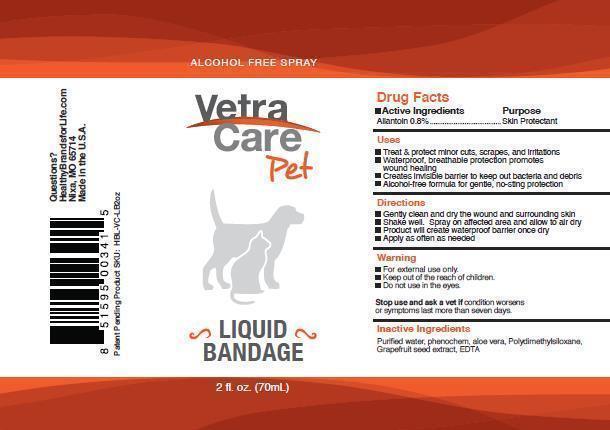
VETERINARY INDICATIONS
Active ingredient Allantoin .8% Skin Protectant
Uses
Treat and protect minor cuts, scrapes, and irritations
waterproof, breathable protection promotes wound healing
Creates invisible barrier to keep out bacteria and debris
Alcohol free formula for gentle, no sting protection
Directions
Gently clean and dry the wound and surrounding skin
shake well. Spray onto affected area and allow to air dry
product will create waterproof barrier once dry
Apply as often as needed
Warning
For external use only
Keep out of the reach of children
Do not use in the eyes
Stop use and ask a vet if condition worsens or symptoms last more than seven days.
Inactive ingredients
Purified water, phenochem, aloe vera, polydimethylsiloxane, grapefruit seed extract, EDTA
-
INGREDIENTS AND APPEARANCE
ALLANTOIN .4%
vetracare pet liquid bandage liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:51098-501(NDC:51098-742) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) Allantoin 4 g in 1000 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) 1 g in 1000 mL water (UNII: 059QF0KO0R) 950 mL in 1000 mL silicon (UNII: Z4152N8IUI) 3 g in 1000 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51098-501-01 60 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2013 Labeler - avadim II, LLC (962520412) Registrant - Avadim II, LLC (962520412) Establishment Name Address ID/FEI Business Operations avadim II, LLC 962520412 manufacture