Label: SENNA-S LAXATIVE WITH STOOL SOFTENER- sennosides tablet
- NDC Code(s): 0761-0910-02
- Packager: Basic Drugs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
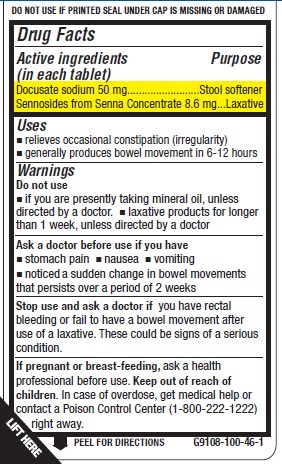
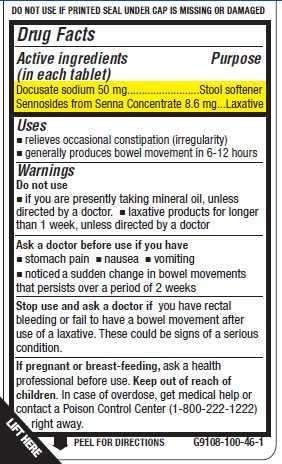
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- if you are taking mineral oil, unless directed by a doctor.
- laxative products for longer than 1 week, unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that persists over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or you fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
-
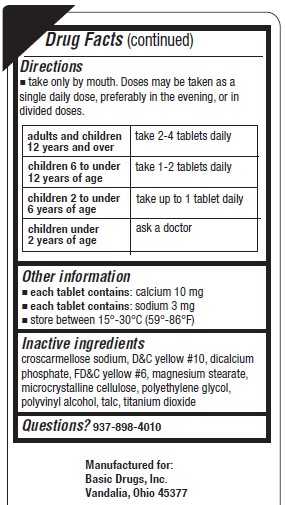
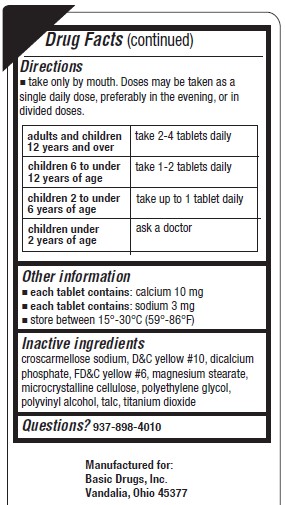
Directions
- take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
adults and children 12 years of age and over 2-4 tablets daily children 6 to under 12 years 1-2 tablets daily children 2 to under 6 years up to tablet daily children under 2 years of age ask a doctor - Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENNA-S LAXATIVE WITH STOOL SOFTENER
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0761-0910 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape ROUND Size 9mm Flavor Imprint Code PH32 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0761-0910-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 12/15/2021 Labeler - Basic Drugs, Inc. (052155082)