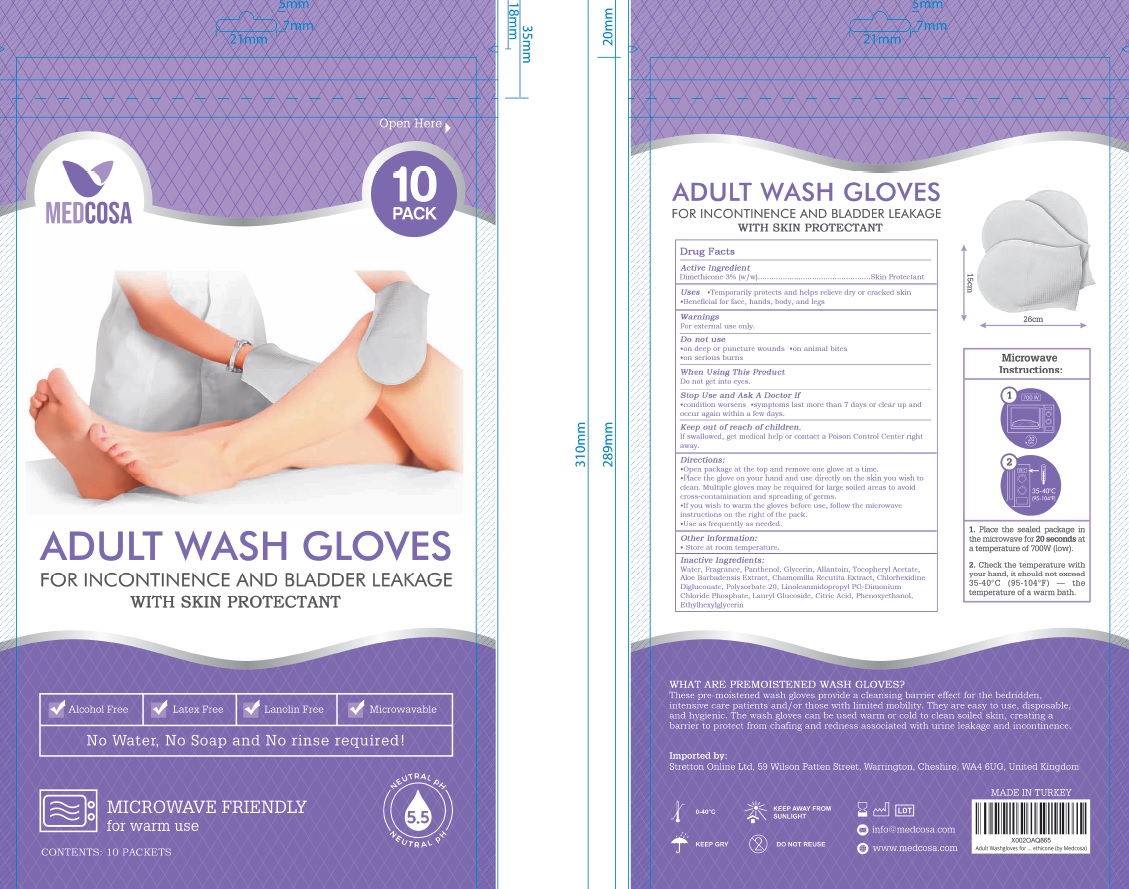
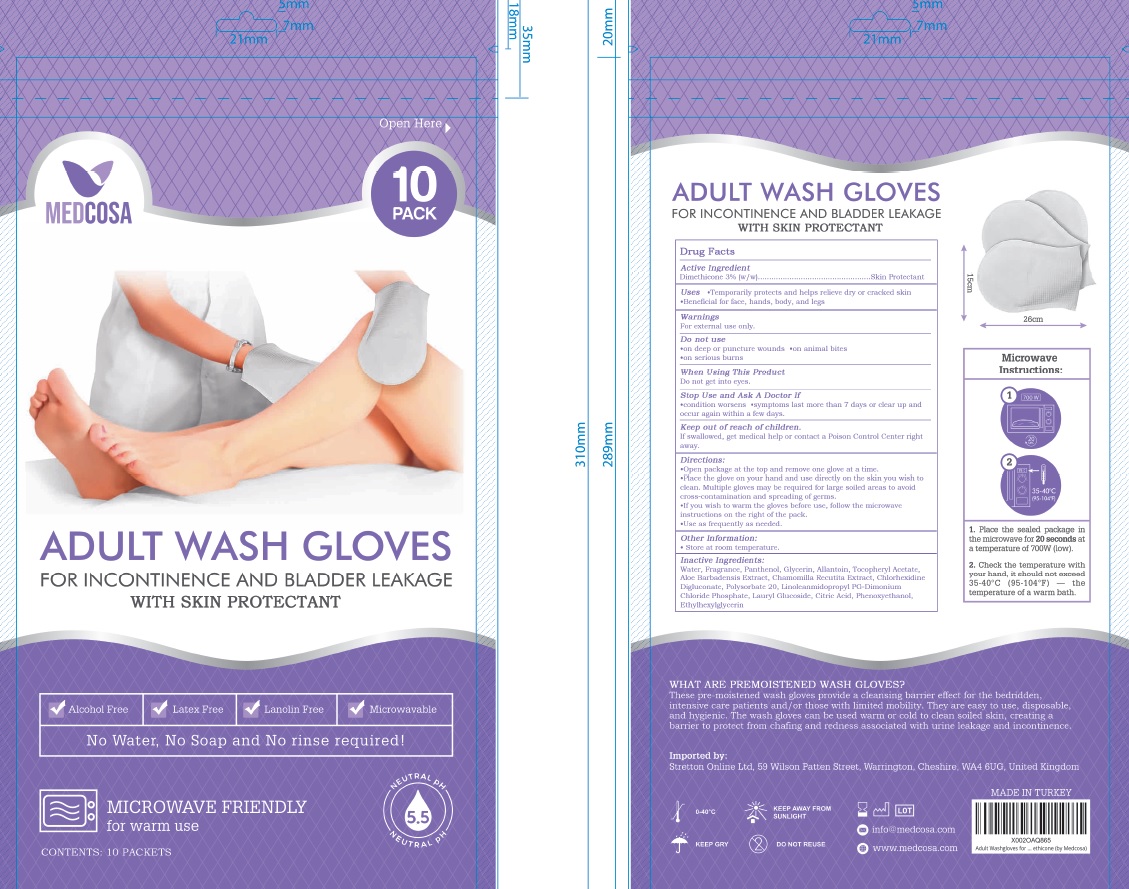
Label: ADULT WASH GLOVES WITH SKIN PROTECTANT- dimethicone cloth
- NDC Code(s): 81310-010-01
- Packager: Stretton Online Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
Open package at the top and remove one glove at a time
Place the glove on your hand and use directly on the skin you wish to clean. Multiple gloves may be required for large soiled areas to avoid cross-contamination and spreading of germs.
If you wish to warm the gloves before use, follow the mircrowave instructions on the right of the pack.
Use as frequently as needed.
- Other information
- Inactive ingredients
- Label Images
-
INGREDIENTS AND APPEARANCE
ADULT WASH GLOVES WITH SKIN PROTECTANT
dimethicone clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81310-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) GLYCERIN (UNII: PDC6A3C0OX) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LINOLEAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: 5Q87K461JO) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) POLYSORBATE 20 (UNII: 7T1F30V5YH) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81310-010-01 20 g in 1 PACKET; Type 0: Not a Combination Product 12/13/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/13/2021 Labeler - Stretton Online Ltd. (220633327)