Label: OCUMEND- arnica montana, ledum palustre kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 76277-211-61 - Packager: Cearna, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 8, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
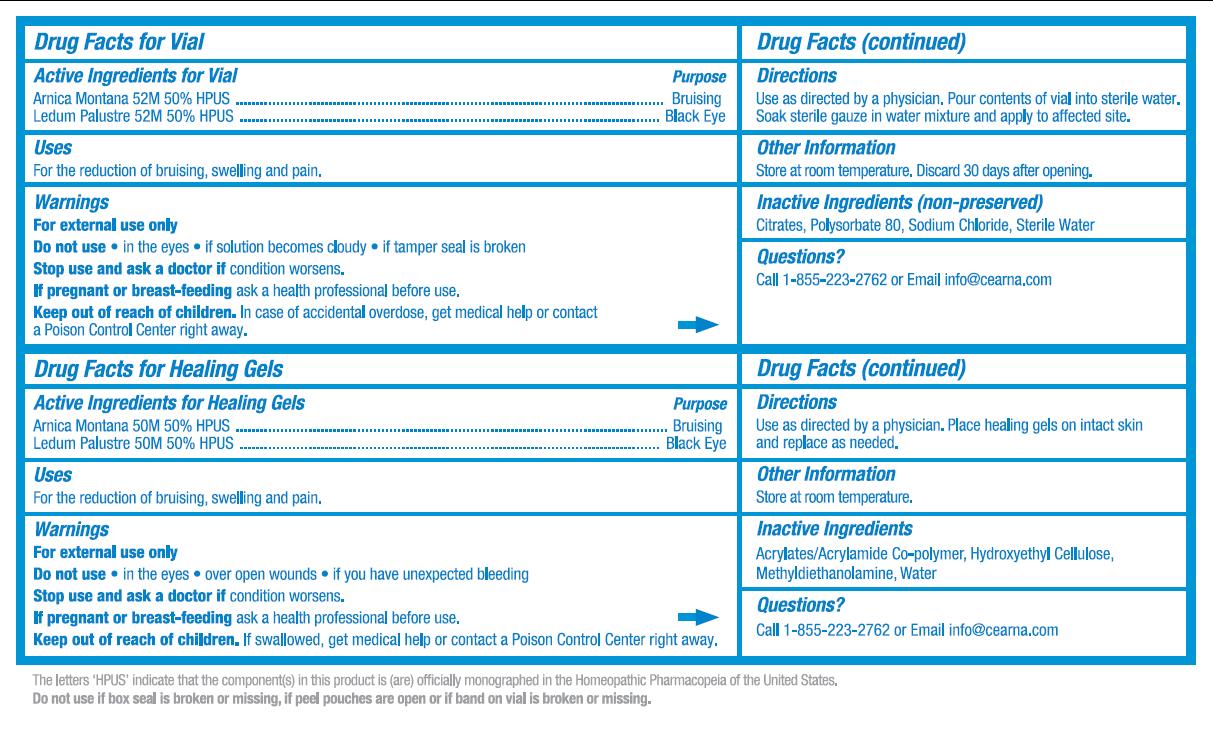
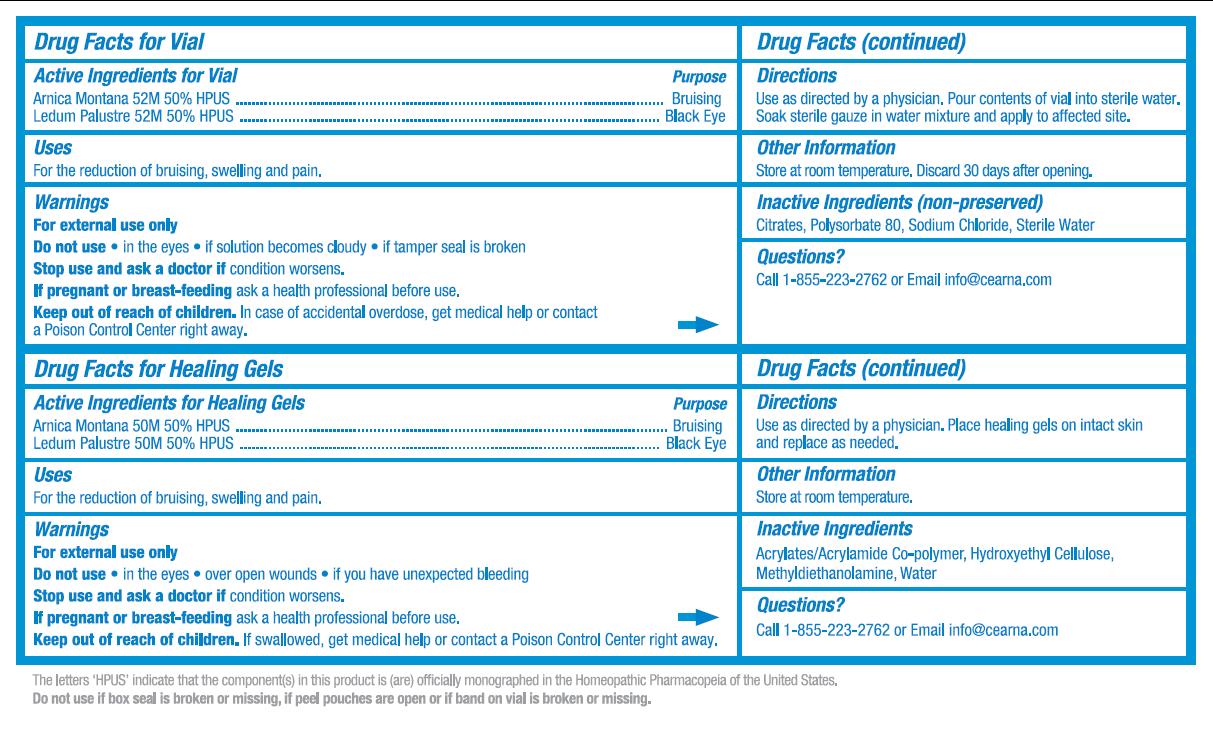
- ACTIVE INGREDIENT
-
PURPOSE
Purpose for Vial:
Arnica Montana 52M 50% HPUS .................................... Bruising
Ledum Palustre 52M 50% HPUS ..................................Black Eye
Purpose for Healing Gels:
Arnica Montana 52M 50% HPUS .................................... Bruising
Ledum Palustre 52M 50% HPUS ..................................Black Eye
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- PREGNANCY OR BREAST FEEDING
-
KEEP OUT OF REACH OF CHILDREN
Children Section for Vial:
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Children Section for Healing Gels:
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OCUMEND
arnica montana, ledum palustre kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76277-211 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76277-211-61 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKAGE 17 Part 2 1 VIAL 15 mL Part 1 of 2 OCUMEND HEALING TWO PACKS
arnica montana, ledum palustre gelProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 52 [hp_M] LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 52 [hp_M] Inactive Ingredients Ingredient Name Strength METHYL DIETHANOLAMINE (UNII: 3IG3K131QJ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/17/2012 Part 2 of 2 OCUMEND STERILE VIAL
arnica montana, ledum palustre liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 52 [hp_M] in 15 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 52 [hp_M] in 15 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/17/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/17/2012 Labeler - Cearna, Inc. (968104609) Registrant - Cearna, Inc. (968104609) Establishment Name Address ID/FEI Business Operations Katecho, Inc. 139791743 manufacture