Label: SPF 30 TINTED MINERAL MOISTURIZER SAND- zinc oxide lotion
SPF 30 TINTED MINERAL MOISTURIZER SHEER- zinc oxide lotion
- NDC Code(s): 55165-0110-1, 55165-0111-1
- Packager: Juice Beauty
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Due to the nature of mineral formulations shake and knead the tube before use. Moisturize daily by applying generously and evenly 15 minutes before sun exposure. May be used over moisturizer or alone.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Pyrus malus (organic apple juice)1, cocos nucifera (organic coconut oil)1, vitis vinifera (organic white grape juice)1, aloe barbadensis (organic aloe leaf juice)1, caprylic/capric triglyceride, sorbitan stearate, ricinus communis (castor seed oil), polyglyceryl-10 laurate, magnesium sulfate, helianthus annuus (organic sunflower seed oil)1, simmondsia chinensis (organic jojoba seed oil)1, tocopherol (Vitamin E), sodium hyaluronate (vegetable hyaluronic acid), phenethyl alcohol, ethylhexylglycerin, citrus reticulata (mandarin) & citrus aurantium (petitgrain) pure essential oils.
- 1
- certified organic ingredient
- Other Information
- Questions?
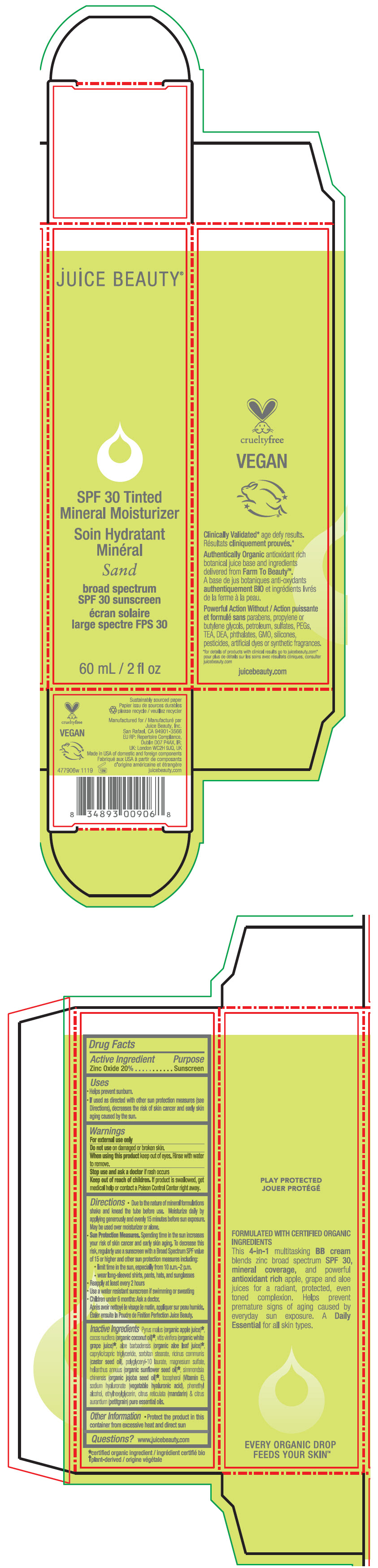
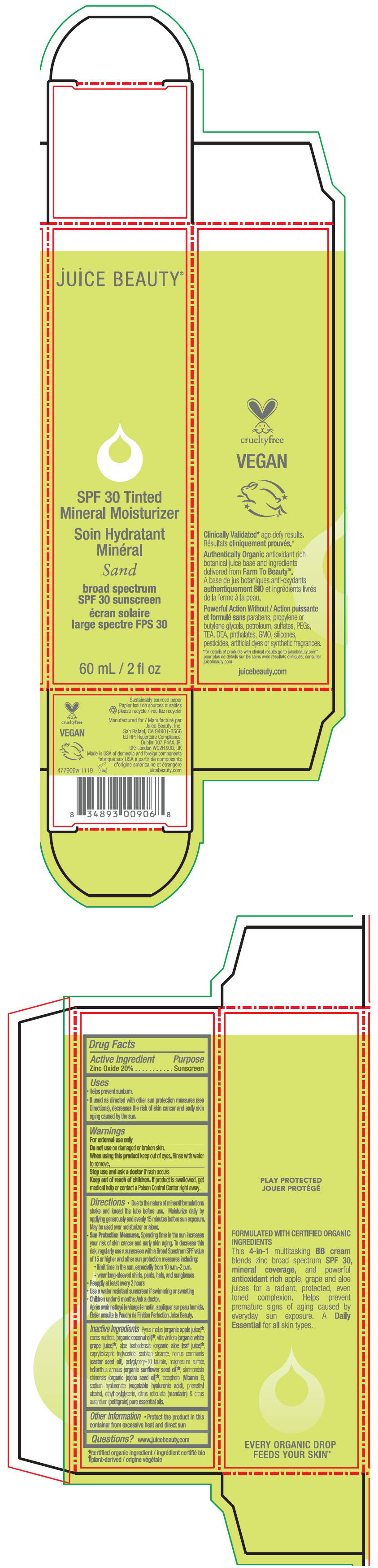
- PRINCIPAL DISPLAY PANEL - 60 mL Tube Carton - Sand
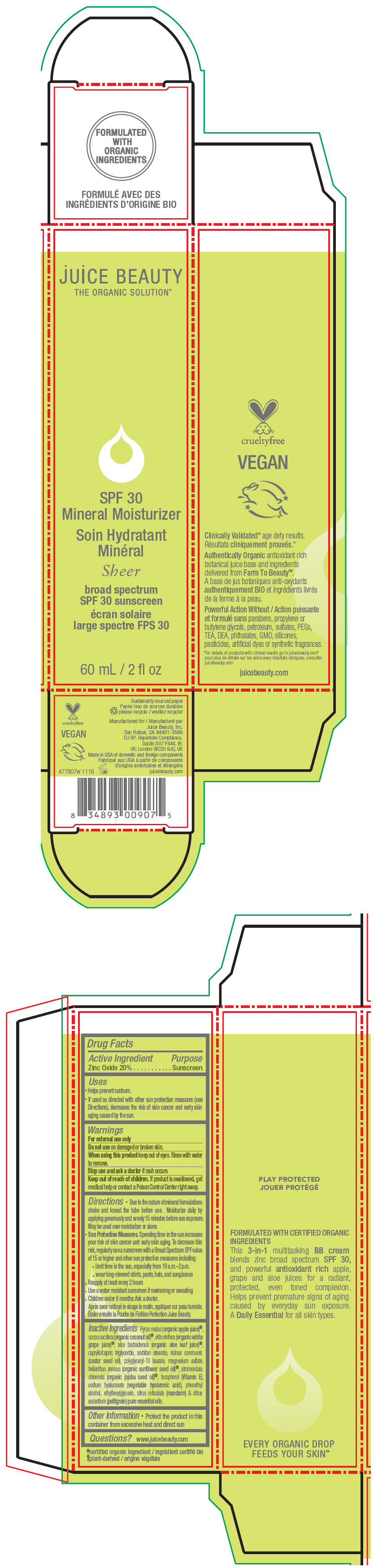
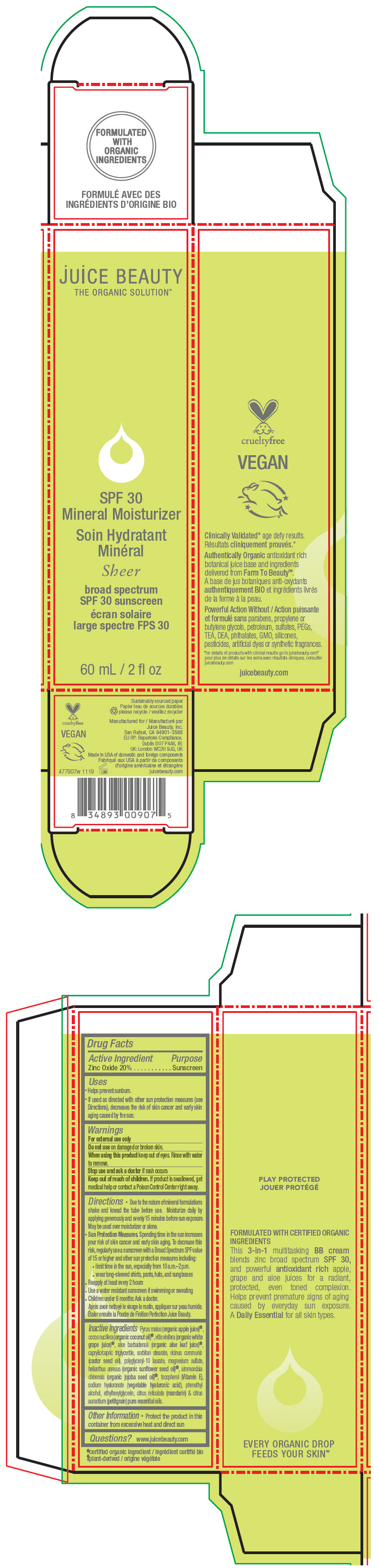
- PRINCIPAL DISPLAY PANEL - 60 mL Tube Carton - Sheer
-
INGREDIENTS AND APPEARANCE
SPF 30 TINTED MINERAL MOISTURIZER SAND
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-0111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength apple juice (UNII: 9871T0PD5P) grape (UNII: 6X543N684K) aloe vera leaf (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) medium-chain triglycerides (UNII: C9H2L21V7U) sorbitan monostearate (UNII: NVZ4I0H58X) castor oil (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) magnesium sulfate, unspecified form (UNII: DE08037SAB) helianthus annuus flowering top (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) hyaluronate sodium (UNII: YSE9PPT4TH) phenylethyl alcohol (UNII: ML9LGA7468) ethylhexylglycerin (UNII: 147D247K3P) mandarin oil (UNII: NJO720F72R) citrus aurantium leafy twig oil (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-0111-1 1 in 1 CARTON 12/10/2012 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 SPF 30 TINTED MINERAL MOISTURIZER SHEER
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55165-0110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength apple juice (UNII: 9871T0PD5P) grape (UNII: 6X543N684K) aloe vera leaf (UNII: ZY81Z83H0X) coconut oil (UNII: Q9L0O73W7L) medium-chain triglycerides (UNII: C9H2L21V7U) sorbitan monostearate (UNII: NVZ4I0H58X) castor oil (UNII: D5340Y2I9G) polyglyceryl-10 laurate (UNII: MPJ2Q8WI8G) magnesium sulfate, unspecified form (UNII: DE08037SAB) helianthus annuus flowering top (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) hyaluronate sodium (UNII: YSE9PPT4TH) phenylethyl alcohol (UNII: ML9LGA7468) ethylhexylglycerin (UNII: 147D247K3P) mandarin oil (UNII: NJO720F72R) citrus aurantium leafy twig oil (UNII: 5K6H1IMT3D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55165-0110-1 1 in 1 CARTON 12/10/2012 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 Labeler - Juice Beauty (263151582)