Label: 100 % MINERAL SPF MINI SET- zinc oxide, titanium dioxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 82384-0020-1, 82384-0021-1, 82384-0022-1, 82384-0023-1, view more82384-0024-1 - Packager: System Beauty, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
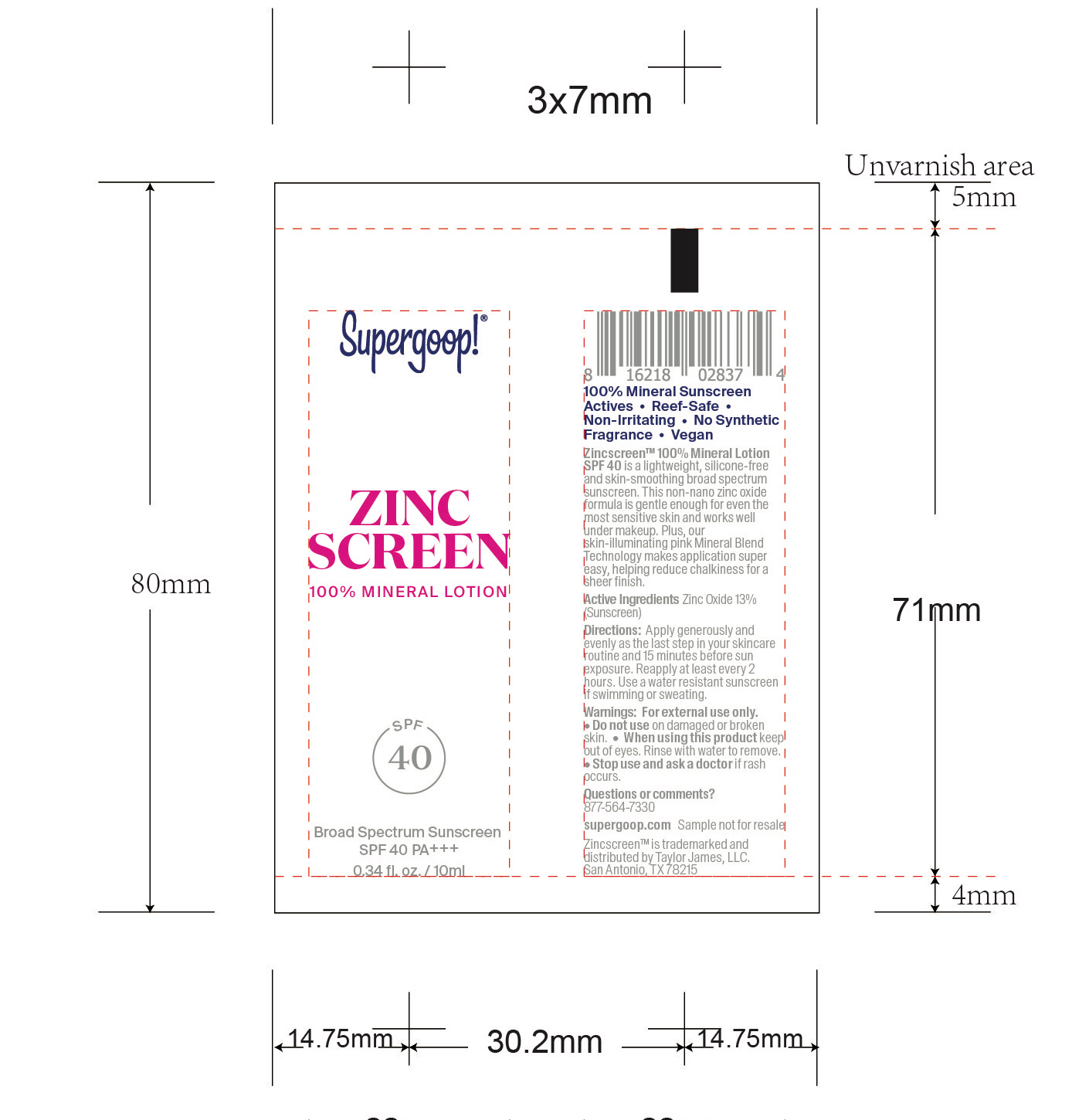
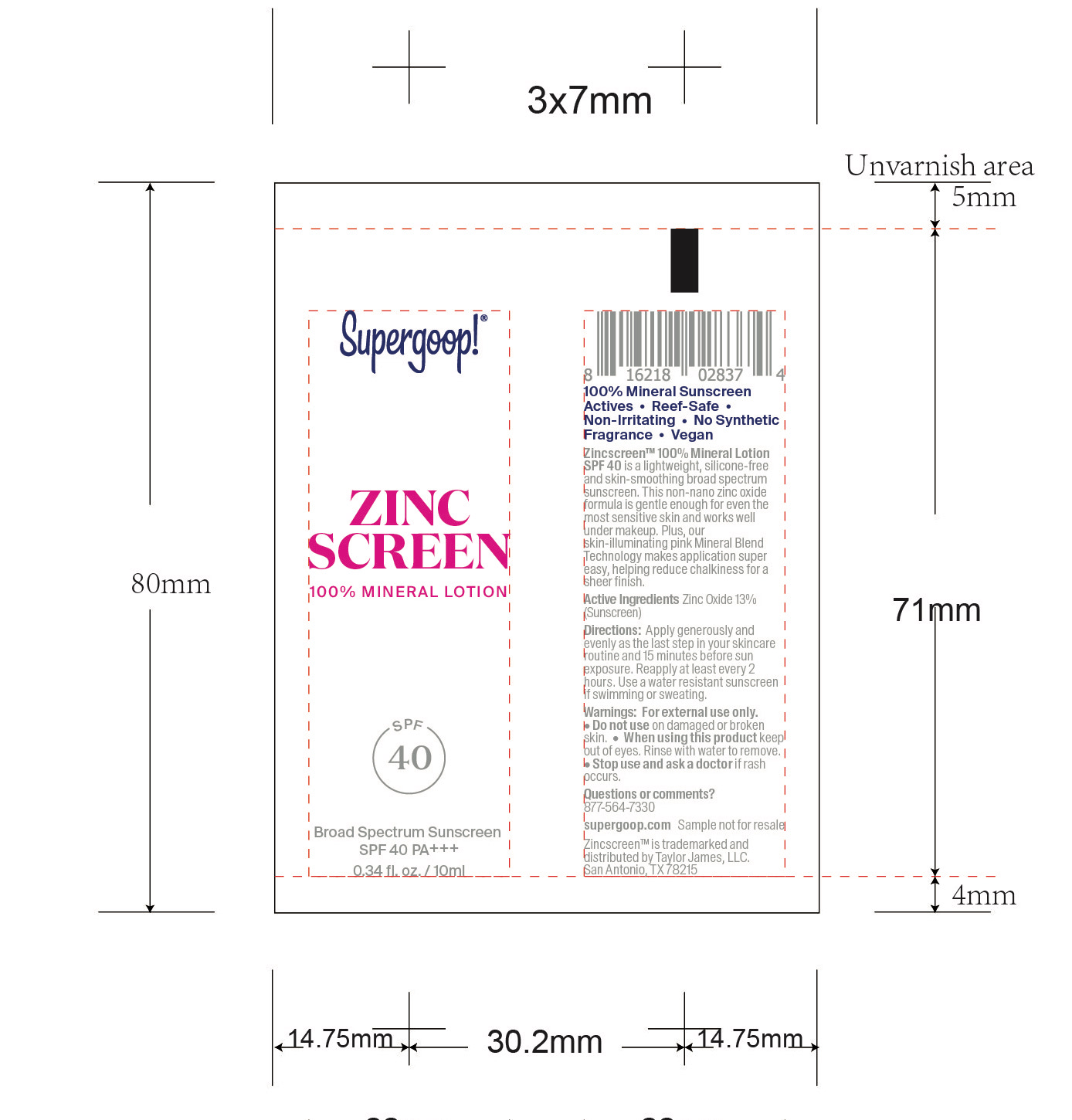
- Zincscreen 100% Mineral Lotion SPF 40 Active
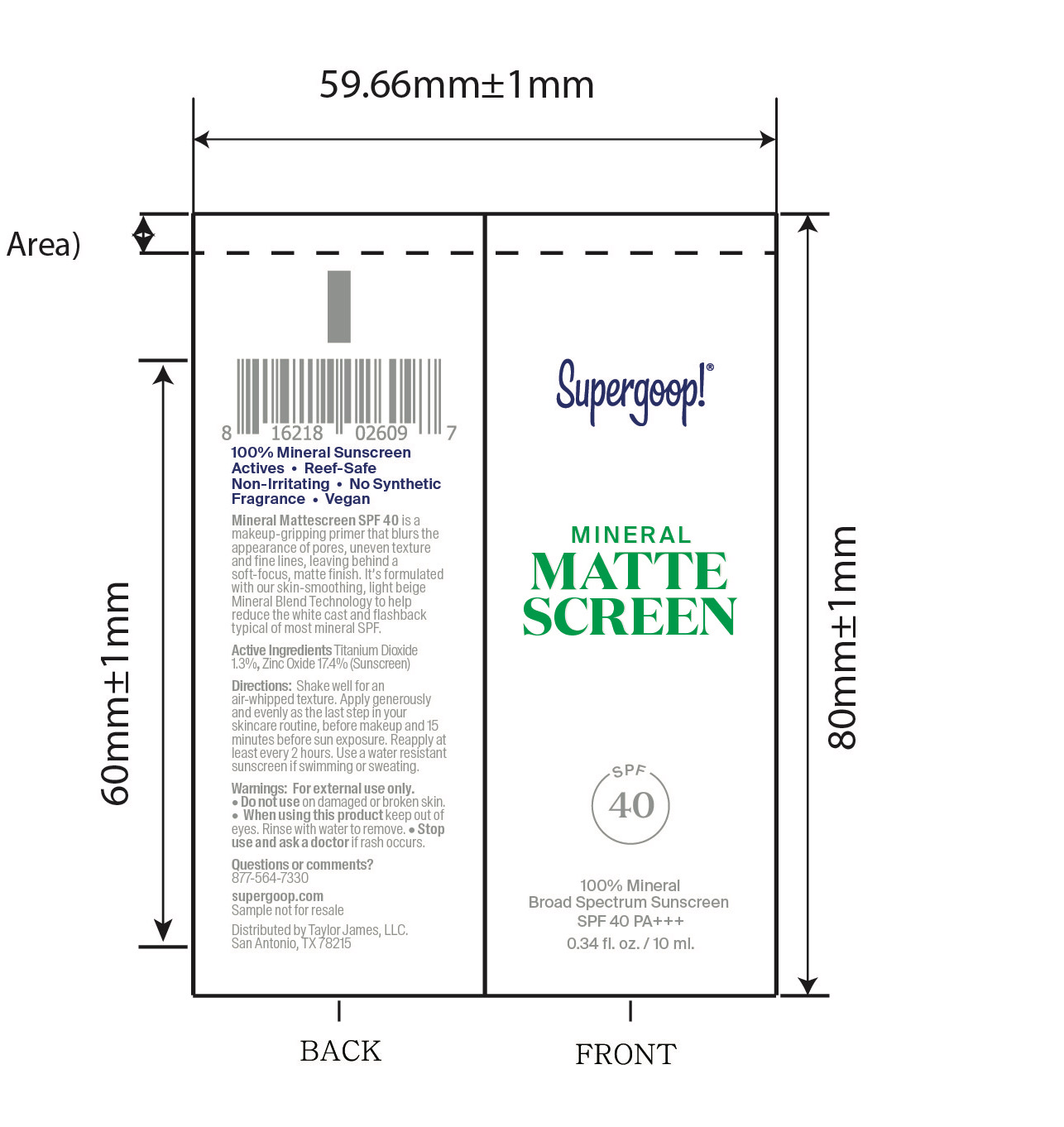
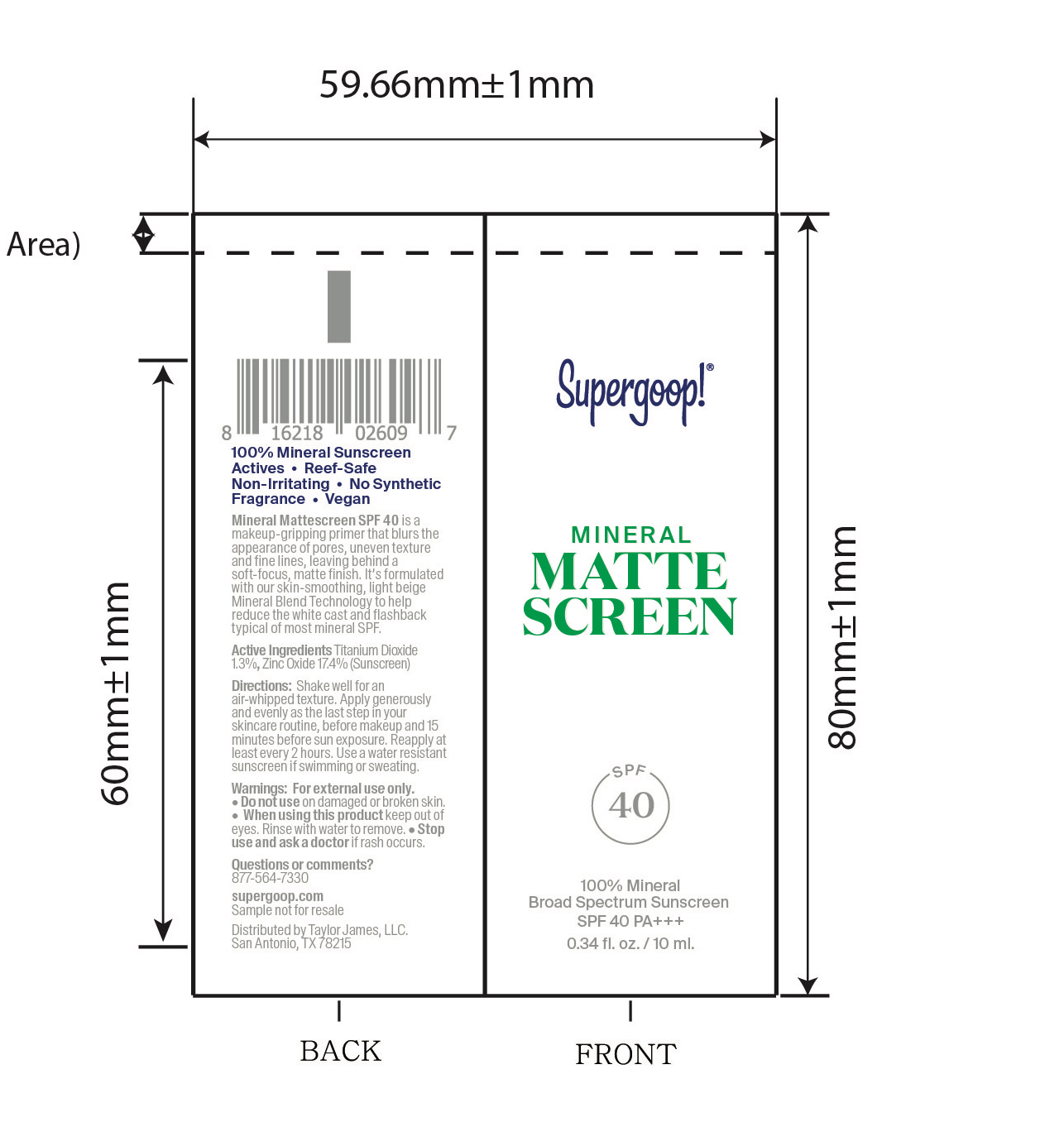
- Mineral Mattescreen SPF 40 active
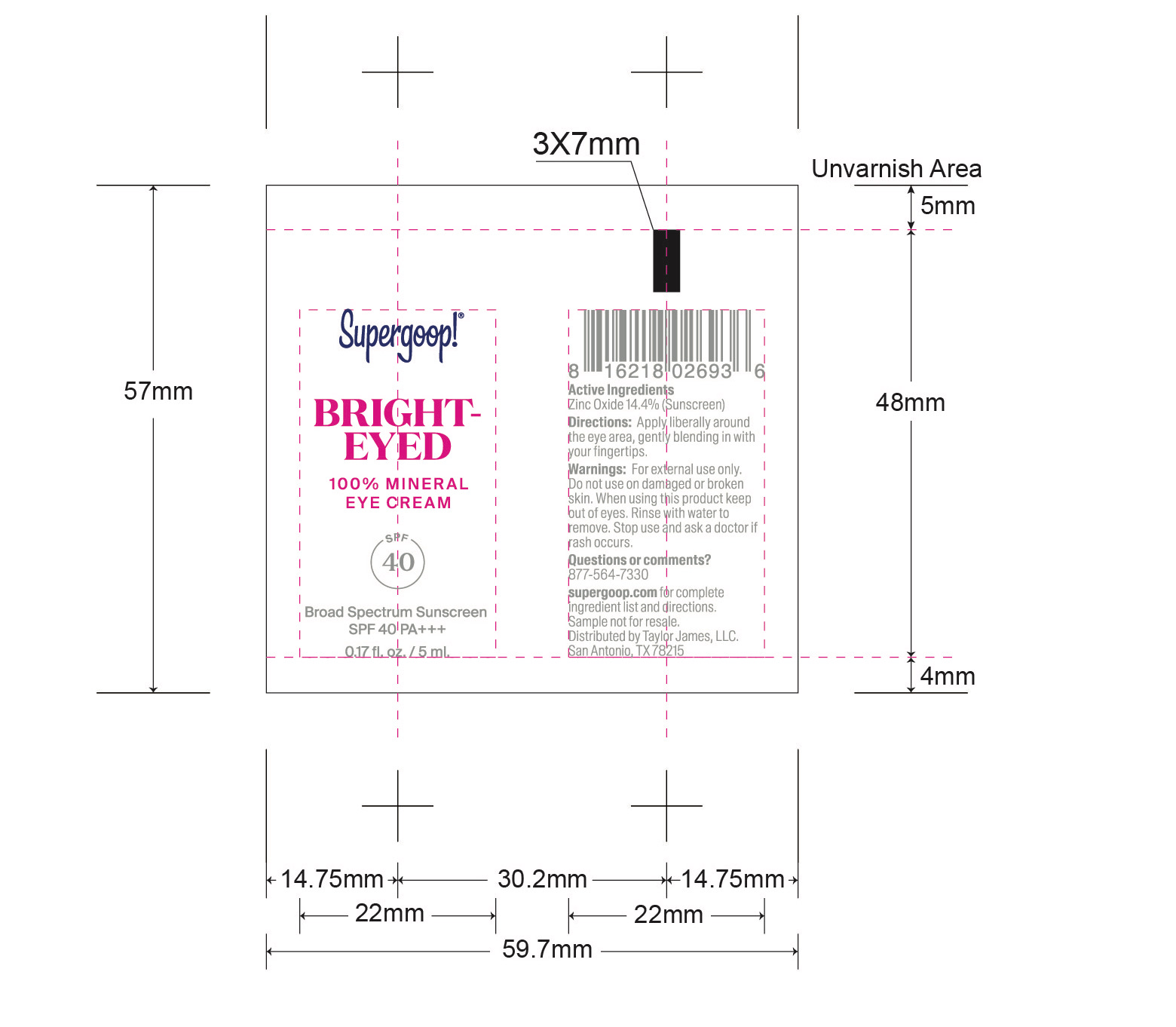
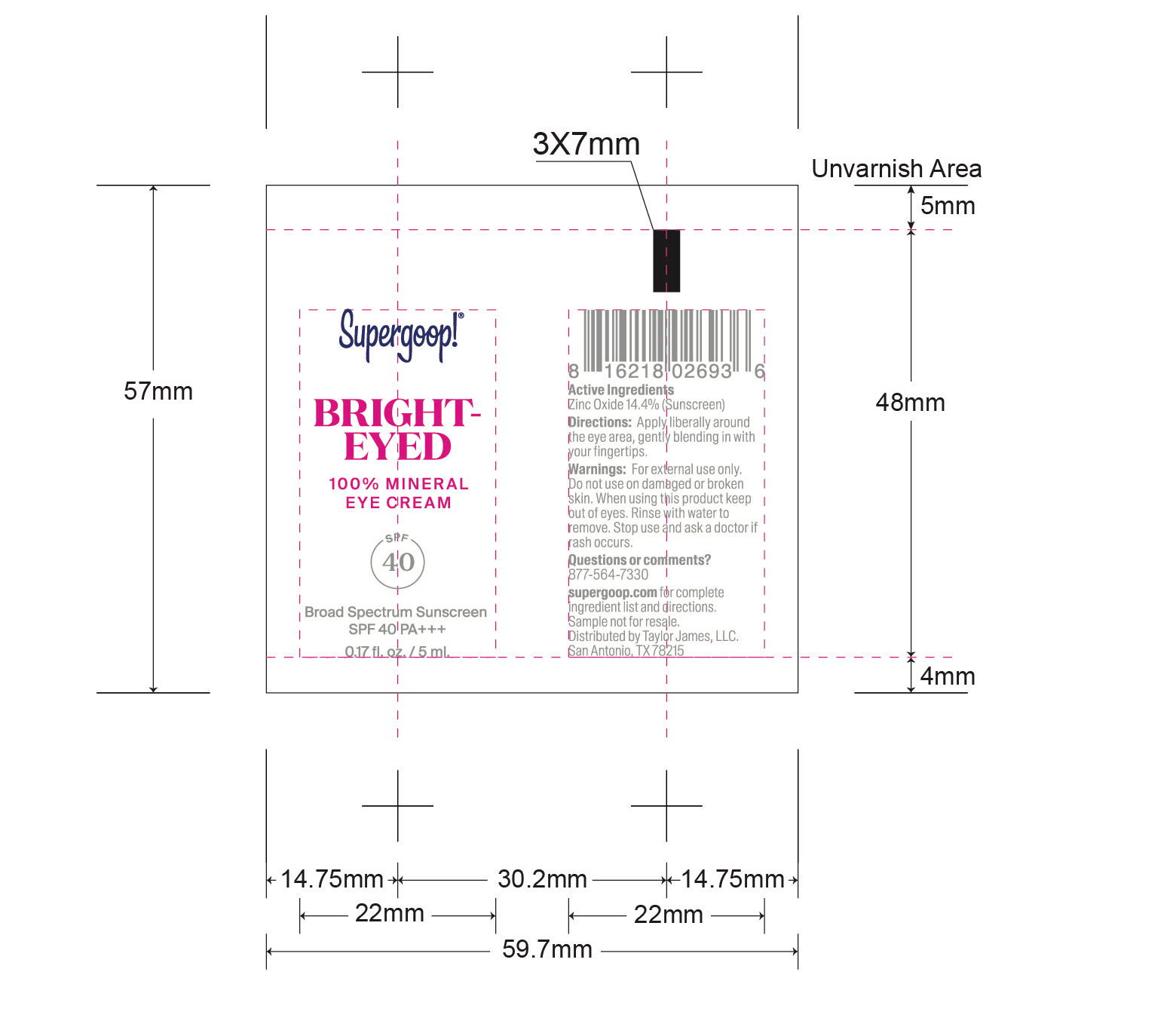
- Bright-Eyed 100% Mineral Eye Cream active
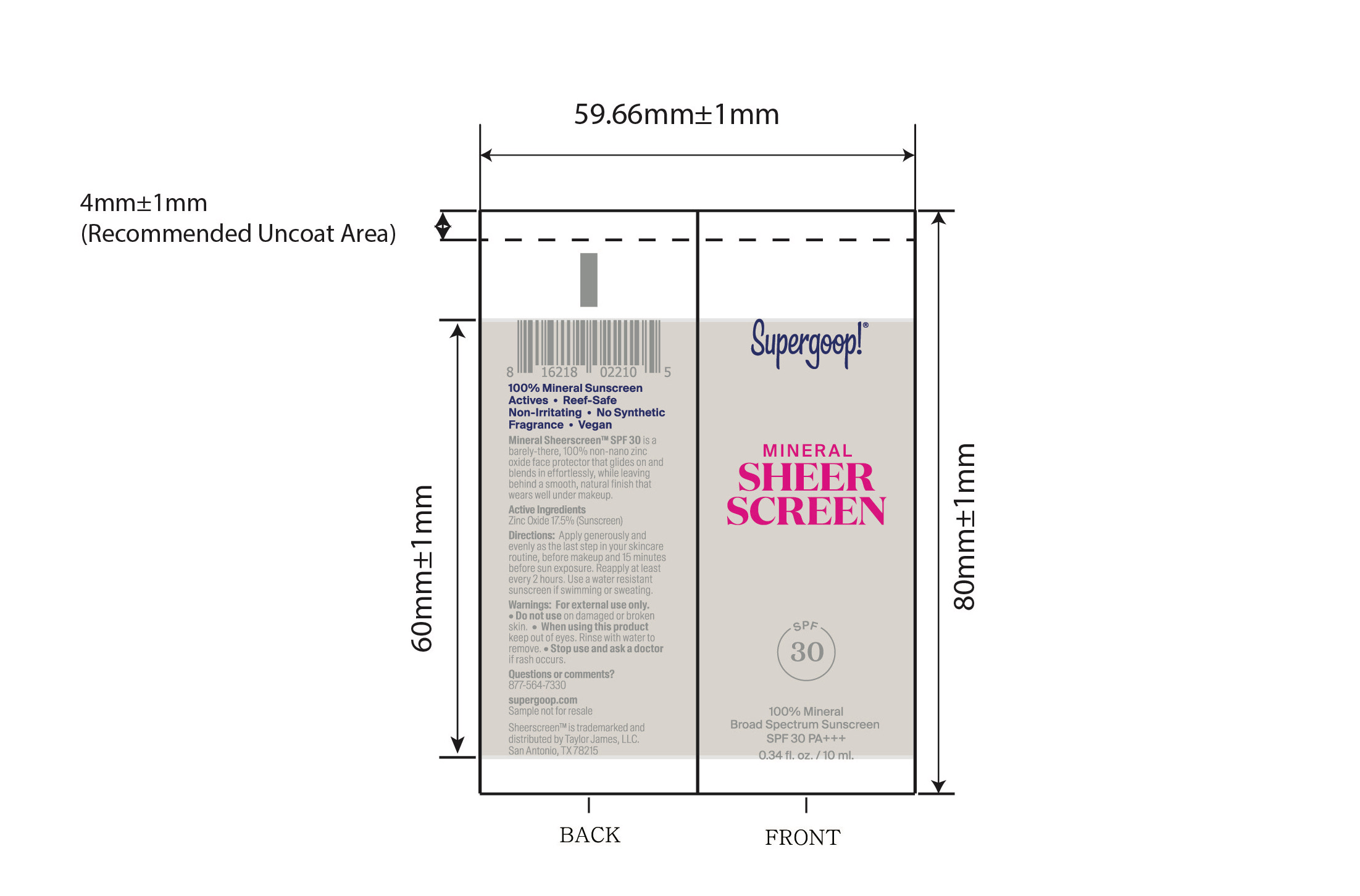
- Mineral Sheerscreen SPF 30 active
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglassess
- Children under 6 months: Ask a doctor.
-
Zincscreen 100% Mineral Lotion SPF 40 Inactive ingredients
Inactive Ingredients
Water, Tetradecane, Cetearyl Nonanoate, Ethylhexyl Palmitate, Caprylic/Capric Triglyceride, Isopropyl Myristate, Glycerin, Polyglyceryl-3 Diisostearate, Mica, Propanediol, Disteardimonium Hectorite, Gluconolactone, Polyglyceryl-6 Polyricinoleate, Brassica Campestris/Aleurites Fordi Oil Copolymer, Polyhydrostearic Acid, Sodium Chloride, Magnesium Sulfate, Fructose, Octyldodecyl Oleate, Tocopherol, Cocos Nucifera (Coconut) Fruit Extract, Sodium Benzoate, Iron oxides (CI 77492), Dodecane, Hexadecane, Titanium Dioxide (CI 77891), Withania Somnifera Flower Extract, Iron Oxides (CI 77491), Vaccinium Angustifolia (Blueberry) Fruit Extract, Silica, Calcium Gluconate
-
Mineral Mattescreen SPF 40 Inactive Ingredients
Inactive Ingredients
Dimethicone, Dimethicone Copolymer, Caprylic/Capric Triglyceride, Methyl Dihydroabietate, Polyhydroxystearic Acid, C9-12 Alkane, Glycerin, Iron Oxides (CI 77492), Ethylhexylglycerin, Water (Aqua), Coco-Caprylate/Caprate, Silica, Stearic Acid, Iron Oxides (CI 77491), Iron Oxides (CI 77499), Hedychium Coronarium Root Extract, Lactobacillus/Arundinaria Gigantea Leaf Ferment Filtrate, Leuconostoc/Radish Root Ferment Filtrate, Titanium Dioxide (CI 77891)
-
Bright-Eyed Mineral Eye Cream SPF 40 inactive ingredients
Inactive Ingredients Water, Caprylic/Capric Triglyceride, Glycerin, Propanediol, Butyloctyl Salicylate, Glyceryl Stearate Citrate, Lauroyl Lysine, Cetyl Esters, Inulin Lauryl CarbamatePolyhydroxystearic Acid, Cetearyl Alcohol, Potassium Cetyl phosophate, Griffonia Simplicifolia Seed Extract, Titanium Dioxide, Olive Oil Polyglyceryl-6 Esters, Mica, Triethoxycaprylylsilane, Microcrystalline cellulose, Glyceryl Glucoside, Hydroxyacetophenone, Polyurethane-79, Diethylhexyl Syringylidenemalonate, Sodium Stearoyl Lactylate, 1,2-Hexanediol, Caprylyl Glycol, Sodium Citrate, Trisodium Ethylenediamine Disuccinate, Camellia Sinensis Leaf Extract, Lactobacillus Ferment Lysate, Punica Granatum Extract, Sodium Citrate, Sodium Stearoyl Glutamate, Xanthan Gum, Cellulose Gum, Iron Oxides, Hedychium Coronarium Root Extract, Lactobacillus Ferment, Caffeine, Leuconostoc/Radish Root Ferment Filtrate, Tin Oxide.
-
Mineral Sheerscreen SPF 30 Inactive ingredients
Inactive ingredients
Water, Isododecane, Caprylic/Capric Triglyceride, Butyloctyl Salicylate, Propanedlol, Glycerin, Squalane, Trioctyldodecyl Citrate, Polyglycerin-3, Polyglyceryl-3 Lactate/Laurate, Polysilicone-11, Cetearyl Alcohol, Glyceryl Stearate, Sodium Stearoyl Glutamate, Butylene Glycol, Xanthan Gum, Polyacrylate Crosspolymer-6, lsostearic Acid, Lecithin, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Caprylhydroxamic Acid, Citric Acid, Aloe Barbadensis Leaf Juice, Lespedeza Capitata Leaf/Stem Extract, Maltodextrin, Sodium Hyaluronate
- 100% Mineral SPF Mini Set
- Zincscreen 100% Mineral SPF 40
- Mineral Mattescreen SPF 40
- Bright-eyed Mineral Eye Cream SPF 40
- Mineral Sheerscreen SPF 30
-
INGREDIENTS AND APPEARANCE
100 % MINERAL SPF MINI SET
zinc oxide, titanium dioxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82384-0020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82384-0020-1 1 in 1 KIT; Type 0: Not a Combination Product 07/30/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 10 mL in 35 Part 2 1 TUBE 10 mL in 35 Part 3 1 TUBE 10 mL in 35 Part 4 1 TUBE 5 mL in 35 Part 1 of 4 MINERAL SHEERSCREEN SPF 30
zinc oxide creamProduct Information Item Code (Source) NDC:82384-0021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.5 g in 100 mL Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ISODODECANE (UNII: A8289P68Y2) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) XANTHAN GUM (UNII: TTV12P4NEE) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) WATER (UNII: 059QF0KO0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIOCTYLDODECYL CITRATE (UNII: 35X8CT063R) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82384-0021-1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/30/2021 Part 2 of 4 MINERAL MATTESCREEN SPF 40
titanium dioxide, zinc oxide creamProduct Information Item Code (Source) NDC:82384-0022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.4 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.3 g in 100 mL Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82384-0022-1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/30/2021 Part 3 of 4 ZINCSCREEN 100% MINERAL SPF 40
zinc oxide lotionProduct Information Item Code (Source) NDC:82384-0023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13 g in 100 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYL PALMITATE (UNII: 2865993309) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) CETEARYL NONANOATE (UNII: F6ZWV2F361) SODIUM CHLORIDE (UNII: 451W47IQ8X) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) FRUCTOSE (UNII: 6YSS42VSEV) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXADECANE (UNII: F8Z00SHP6Q) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) OCTYLDODECYL OLEATE (UNII: MCA43PK7MH) CALCIUM GLUCONATE (UNII: SQE6VB453K) SODIUM BENZOATE (UNII: OJ245FE5EU) TETRADECANE (UNII: 03LY784Y58) GLUCONOLACTONE (UNII: WQ29KQ9POT) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) TOCOPHEROL (UNII: R0ZB2556P8) COCONUT (UNII: 3RT3536DHY) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) GLYCERIN (UNII: PDC6A3C0OX) WITHANIA SOMNIFERA FLOWER (UNII: 2HZ95R7082) FERRIC OXIDE RED (UNII: 1K09F3G675) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DODECANE (UNII: 11A386X1QH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MICA (UNII: V8A1AW0880) PROPANEDIOL (UNII: 5965N8W85T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82384-0023-1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/30/2021 Part 4 of 4 BRIGHT- EYED 100% MINERAL EYE CREAM
zinc oxide creamProduct Information Item Code (Source) NDC:82384-0024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.4 g in 100 mL Inactive Ingredients Ingredient Name Strength GRIFFONIA SIMPLICIFOLIA SEED (UNII: LUS5142TMY) MICA (UNII: V8A1AW0880) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SODIUM CITRATE (UNII: 1Q73Q2JULR) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) PROPANEDIOL (UNII: 5965N8W85T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) CAFFEINE (UNII: 3G6A5W338E) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETYL ESTERS WAX (UNII: D072FFP9GU) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) STANNIC OXIDE (UNII: KM7N50LOS6) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) LAUROYL LYSINE (UNII: 113171Q70B) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) XANTHAN GUM (UNII: TTV12P4NEE) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) OLIVE OIL POLYGLYCERYL-6 ESTERS (UNII: 4KDO9AFM9I) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82384-0024-1 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/30/2021 Labeler - System Beauty, LLC (809198398)