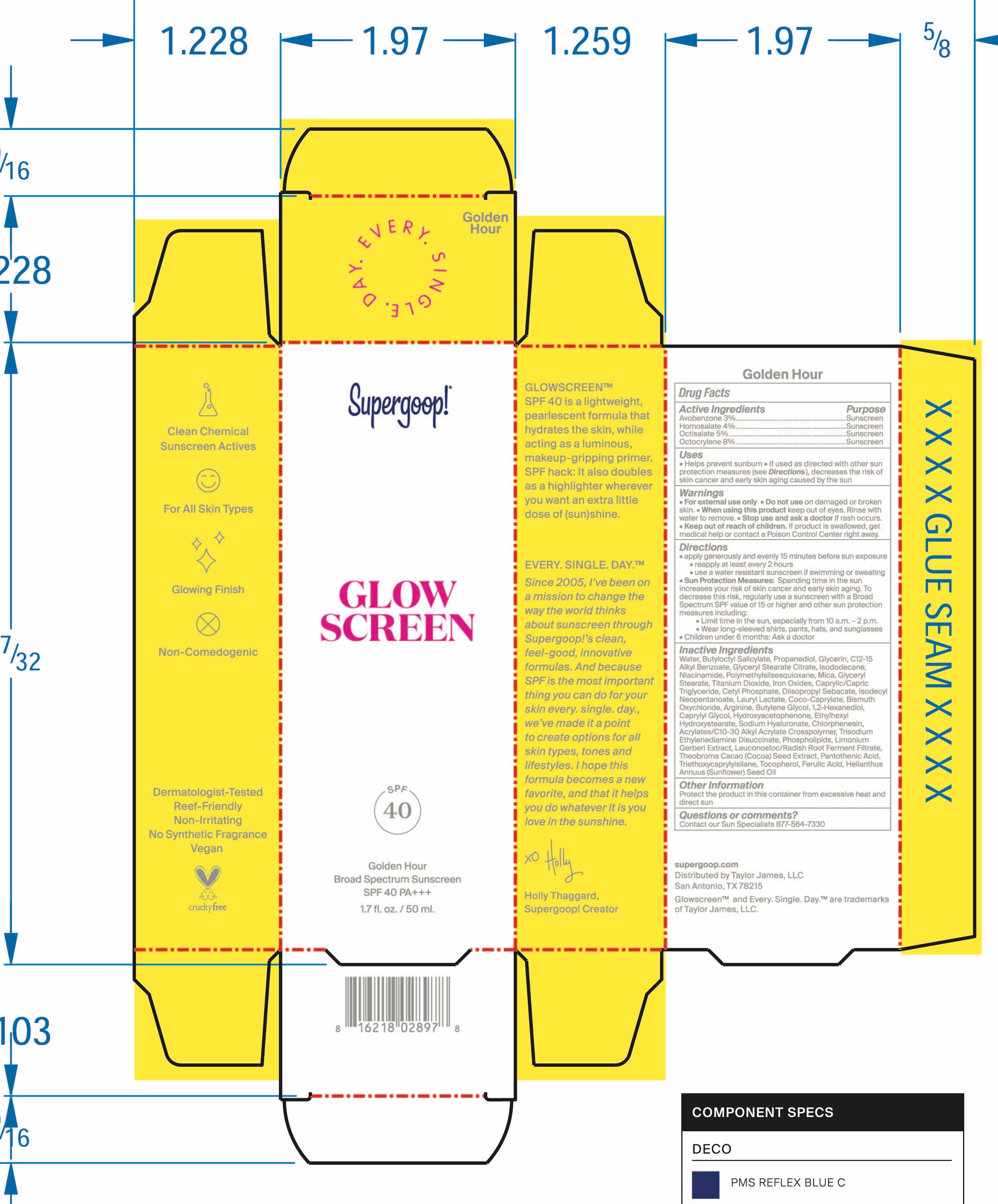
Label: SUPERGOOP GLOW SCREEN SPF 40 SHADE 2 GOLDEN HOUR- broad spectrum sunscreen cream
-
NDC Code(s):
75936-601-01,
75936-601-02,
75936-601-03,
75936-601-04, view more75936-601-05, 75936-601-06
- Packager: Supergoop LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep Out Of Reach of Children
- Indications and Usage
- Warnings
-
Dosage and Administration
Apply generously and evenly 15 minutes before sun exposure
rRapply at least every 2 hours
Use a water resistant Sunscreen if swimming or sweating
Sun Protection Measures Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a
Broad Spectrum SPF value of 15 or higher and other sun protection measures
including Limit time in the sun, especially from 10 a.m. - 2 p.m. Wear long-sleeved shirts, pants, hats, and sun glasses
Children under 6 months: Ask a doctor -
Inactive Ingrediens
Water, Butyloctyl Salicylate , Propanediol, Glycerin, C12-C15 Alkyl Benzoate, Blyceryl Stearate Citrate, Isododecane, Niacinamide, Polymethylsilsesquioxane, Mica, Glyceryl Stearate, Titanium Dioxide, Iron Oxides, Caprylic/Capric Triglyceride, Cetyl Phosphate, Diisopropyl Sabacate, Isododecyl Neopentanoate, Lauryl Lactate, Coco-Caprylate, Bismuth Oxychloride, Arginine, Butylene Glycol, 1,2-Hexanediol, Caprylyl Glycol, Hydroxyacetophenone, Ethylhexyl Hudroxystearate, Sodium Hyaluronate, Chlorphenesin, Acrylates/C10-C30 Alkyl Acrylate Crosspolymer, Trisodium Ethylenediamine Disuccinate, Phospholipds, Limonium Gerberi Extract, Leuconostoc/Radish Root Ferment Filtrate, Theobroma Cacao (Cocoa) Seed Extract, Pantothenic Acid, Triethoxycprylylsilane, Tocopherol, Ferulic Acid, Helianthus Annus (Sunflower) Seed Oil
- Package Label/PDP
-
INGREDIENTS AND APPEARANCE
SUPERGOOP GLOW SCREEN SPF 40 SHADE 2 GOLDEN HOUR
broad spectrum sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4 mg in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8 mg in 100 mL Inactive Ingredients Ingredient Name Strength LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) PANTOTHENIC ACID (UNII: 19F5HK2737) FERULIC ACID (UNII: AVM951ZWST) ETHYLHEXYL HYDROXYSTEARATE (UNII: B7I80BVV5E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) SUNFLOWER OIL (UNII: 3W1JG795YI) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) COCO-CAPRYLATE (UNII: 4828G836N6) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHLORPHENESIN (UNII: I670DAL4SZ) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CETYL PHOSPHATE (UNII: VT07D6X67O) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) LAURYL LACTATE (UNII: G5SU0BFK7O) ARGININE (UNII: 94ZLA3W45F) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) GLYCERIN (UNII: PDC6A3C0OX) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) NIACINAMIDE (UNII: 25X51I8RD4) BROWN IRON OXIDE (UNII: 1N032N7MFO) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) ISODODECANE (UNII: A8289P68Y2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) WATER (UNII: 059QF0KO0R) C12-20 ALKYL BENZOATE (UNII: Y15I6XI14C) LIMONIUM GERBERI WHOLE (UNII: 2J5K7YCF9F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-601-02 1 in 1 BOX 12/20/2021 1 NDC:75936-601-01 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:75936-601-03 10 mL in 1 TUBE; Type 0: Not a Combination Product 12/20/2021 3 NDC:75936-601-04 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 12/20/2021 4 NDC:75936-601-06 1 in 1 BOX 12/20/2021 4 NDC:75936-601-05 20 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/20/2021 Labeler - Supergoop LLC (117061743)