Label: THE INKEY LIST ACNE CLEARING MOISTURIZER- sulfur cream
- NDC Code(s): 81136-024-01
- Packager: Brand Evangelists for Beauty Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
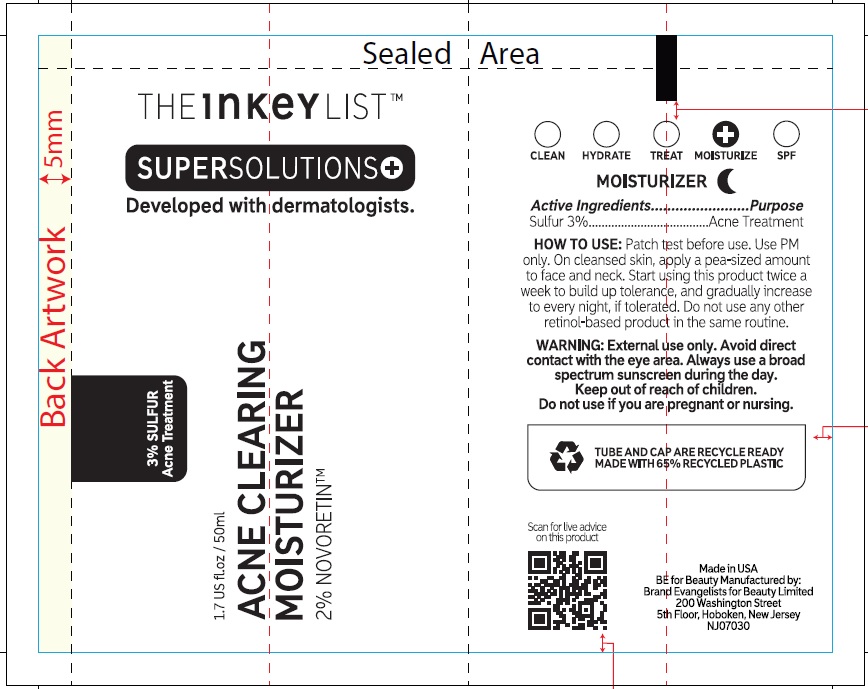
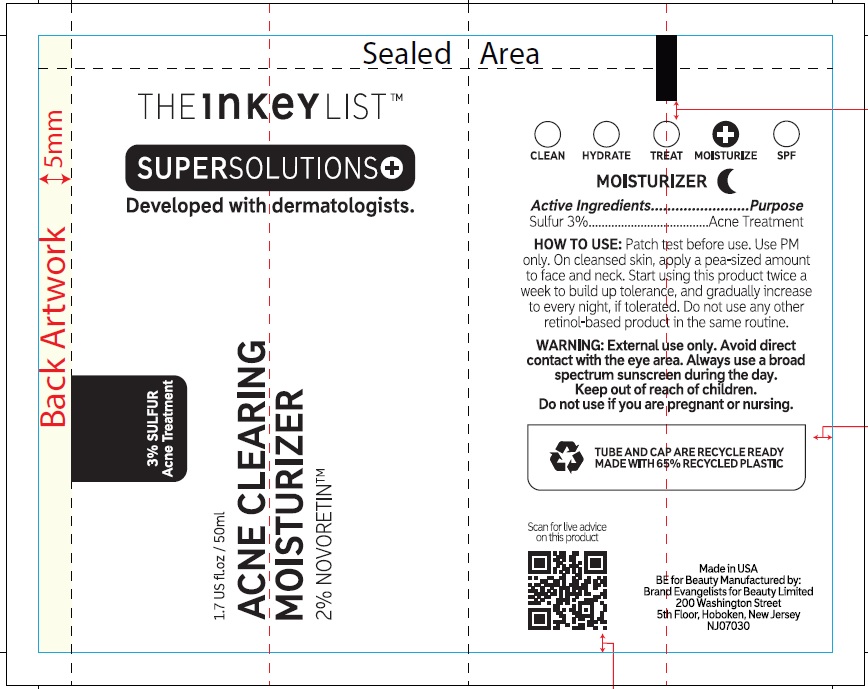
- Active ingredients
- Uses
- Warnings
-
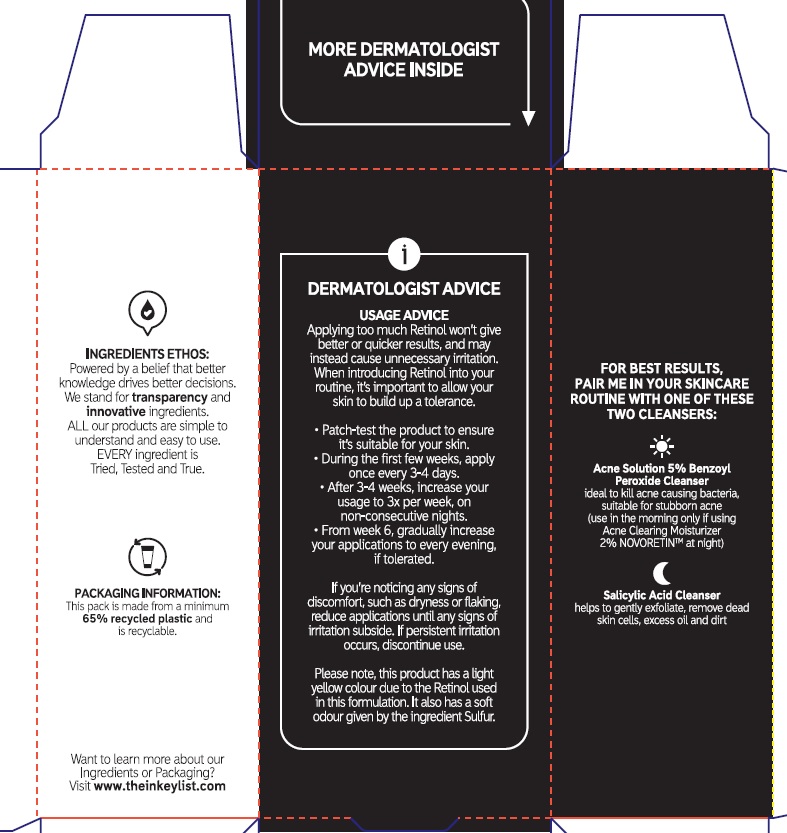
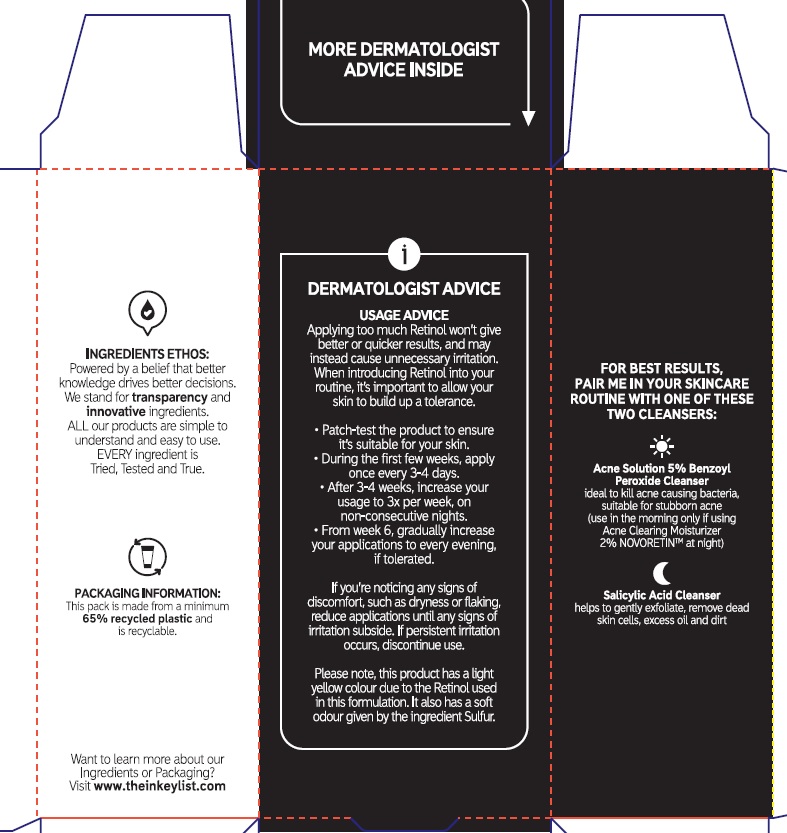
Directions
Sensitivity Test for a New User.
Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated below
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occurs, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to to once a day or every other day.
-
Inactive ingredients
Aqua (Water), Glycerin, Octyldodecanol, Caprylic/Capric Triglyceride, Sodium Ascorbyl Phosphate, Arachidyl Alcohol, Pentaerythrityl Distearate, Squalane, Glyceryl Stearate SE, Citronellyl Methylcrotonate, Behenyl Alcohol, Acacia Senegal Gum, Arachidyl Glucoside, Phenoxyethanol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Panthenol, Xanthan Gum, Benzyl Alcohol, Citric Acid, Retinol, Sodium Hyaluronate, Ethylhexylglycerin, Pentylene Glycol, Sodium Metabisulfite, Pistacia Lentiscus (Mastic) Gum, Dehydroacetic Acid, Lecithin, Glucose, Tocopherol, Glyceryl Caprylate.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
THE INKEY LIST ACNE CLEARING MOISTURIZER
sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81136-024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) PENTAERYTHRITYL DISTEARATE (UNII: 697WOT8HNB) SQUALANE (UNII: GW89575KF9) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CITRONELLYL METHYLCROTONATE (UNII: K61O222P3D) DOCOSANOL (UNII: 9G1OE216XY) ACACIA (UNII: 5C5403N26O) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PANTHENOL (UNII: WV9CM0O67Z) XANTHAN GUM (UNII: TTV12P4NEE) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) RETINOL (UNII: G2SH0XKK91) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM METABISULFITE (UNII: 4VON5FNS3C) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81136-024-01 1 in 1 CARTON 07/01/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/01/2023 Labeler - Brand Evangelists for Beauty Limited (222990724)