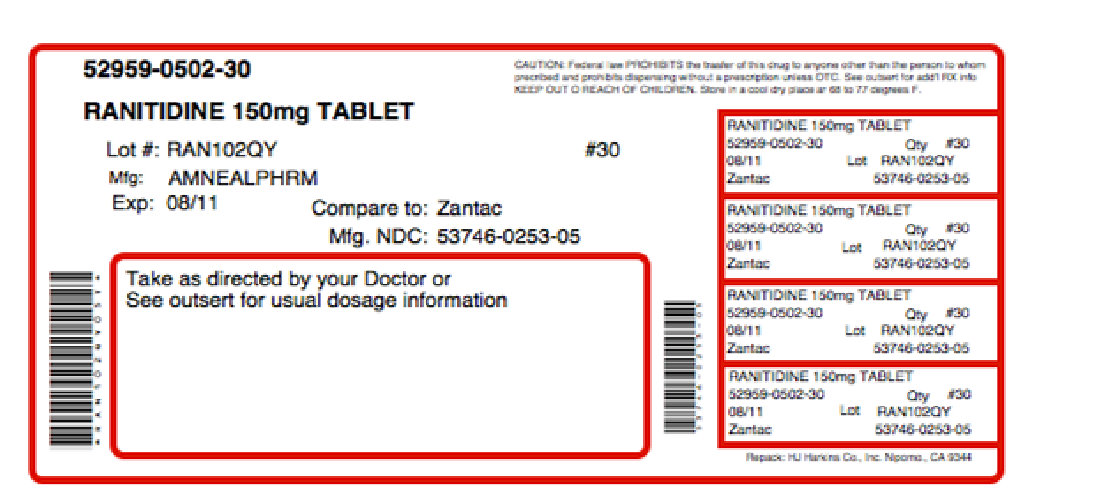
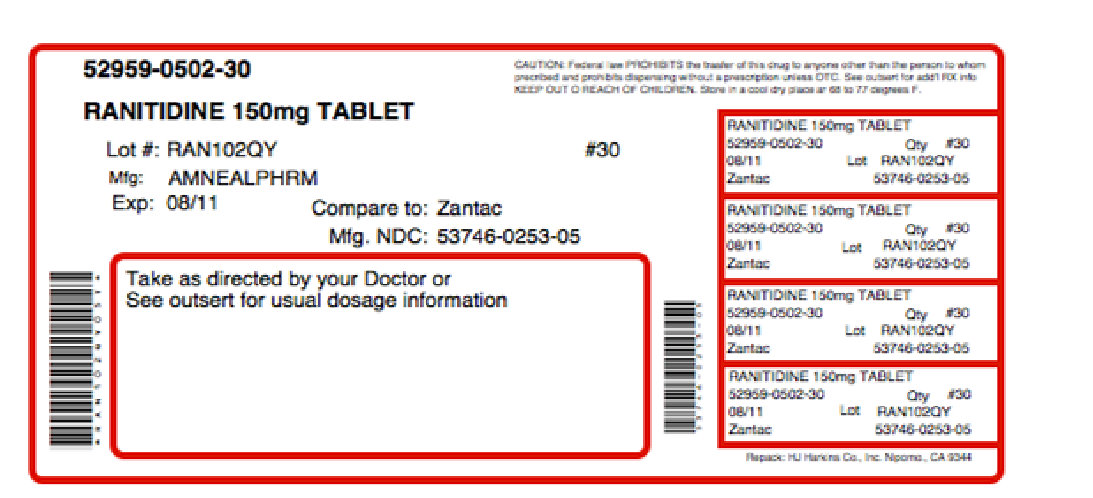
Label: SENTRADINE- ranitidine hydrochloride, choline kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-502-30, 68405-033-26 - Packager: Physician Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION
The active ingredient in Ranitidine Tablets, USP 150 mg and Ranitidine Tablets, USP 300 mg is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structure:
The empirical formula is C13H22N4O3S·HCl, representing a molecular weight of 350.87. Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfurlike odor.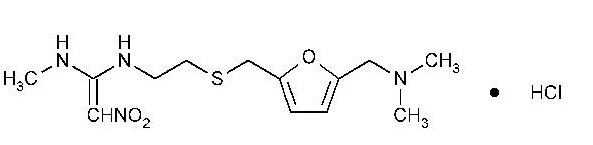
Each Ranitidine Tablets, USP 150 mg for oral administration contains 167.4 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide, triethyl citrate and FD C Yellow #6.
Each Ranitidine Tablets, USP 300 mg for oral administration contains 334.8 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide, triethyl citrate and D C Yellow #10. -
CLINICAL PHARMACOLOGY
Ranitidine Tablets, USP is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP does not lower serum Ca++ in hypercalcemic states. Ranitidine Tablets, USP is not an anticholinergic agent.
Pharmacokinetics: Absorption: Ranitidine Tablets, USP is 50% absorbed after oral administration, compared to an intravenous (IV) injection with mean peak levels of 440 to 545 ng/mL occurring 2 to 3 hours after a 150-mg dose. Absorption is not significantly impaired by the administration of food or antacids. Propantheline slightly delays and increases peak blood levels of Ranitidine Tablets, USP, probably by delaying gastric emptying and transit time. In one study, simultaneous administration of high-potency antacid (150 mmol) in fasting subjects has been reported to decrease the absorption of Ranitidine Tablets, USP.
Distribution: The volume of distribution is about 1.4 L/kg. Serum protein binding averages 15%.
Metabolism: In humans, the N-oxide is the principal metabolite in the urine; however, this amounts to <4% of the dose. Other metabolites are the S-oxide (1%) and the desmethyl ranitidine (1%). The remainder of the administered dose is found in the stool. Studies in patients with hepatic dysfunction (compensated cirrhosis) indicate that there are minor, but clinically insignificant, alterations in ranitidine half-life, distribution, clearance, and bioavailability.
Excretion: The principal route of excretion is the urine, with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. Renal clearance is about 410 mL/min, indicating active tubular excretion. The elimination half-life is 2.5 to 3 hours. Four patients with clinically significant renal function impairment (creatinine clearance 25 to 35 mL/min) administered 50 mg of ranitidine intravenously had an average plasma half-life of 4.8 hours, a ranitidine clearance of 29 mL/min, and a volume of distribution of 1.76 L/kg. In general, these parameters appear to be altered in proportion to creatinine clearance (see DOSAGE AND ADMINISTRATION).
Geriatrics: The plasma half-life is prolonged and total clearance is reduced in the elderly population due to a decrease in renal function. The elimination half-life is 3 to 4 hours. Peak levels average 526 ng/mL following a 150-mg twice daily dose and occur in about 3 hours (see PRECAUTIONS: Geriatric Use and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function).
Pediatrics: There are no significant differences in the pharmacokinetic parameter values for ranitidine in pediatric patients (from 1 month up to 16 years of age) and healthy adults when correction is made for body weight. The average bioavailability of ranitidine given orally to pediatric patients is 48% which is comparable to the bioavailability of ranitidine in the adult population. All other pharmacokinetic parameter values (t1/2 , Vd, and CL) are similar to those observed with intravenous ranitidine use in pediatric patients. Estimates of Cmax and Tmax are displayed in Table 1.
Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing Population (age) N Dosage Form
(dose)Cmax
(ng/mLTmax
(hours)Gastric or duodenal ulcer
(3.5 to 16 years)12 Tablets|
(1 to 2 mg/kg)54 to 492 2.0 Otherwise healthy requiring Ranitidine
(0.7 to 14 years, Single dose)10 Syrup
(2 mg/kg)244 1.61 Otherwise healthy requiring Ranitidine
(0.7 to 14 years, Multiple dose)10 Syrup
(2 mg/kg)320 1.66 Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION: Pediatric Use).
Pharmacodynamics: Serum concentrations necessary to inhibit 50% of stimulated gastric acid secretion are estimated to be 36 to 94 ng/mL. Following a single oral dose of 150 mg, serum concentrations of Ranitidine Tablets, USP are in this range up to 12 hours. However, blood levels bear no consistent relationship to dose or degree of acid inhibition.
Antisecretory Activity: 1. Effects on Acid Secretion: Ranitidine Tablets, USP inhibits both daytime and nocturnal basal gastric acid secretions as well as gastric acid secretion stimulated by food, betazole, and pentagastrin, as shown in Table 2.
Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion Time After
Dose, h% Inhibition of Gastric Acid Output by Dose, mg 75-80 100 150 200 Basal Up to 4 99 95 Nocturnal Up to 13 95 96 92 Betazole Up to 3 97 99 Pentagastin Up to 5 58 72 72 80 Meal Up to 3 73 79 95 It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are most difficult to suppress.
2. Effects on Other Gastrointestinal Secretions:
Pepsin: Oral Ranitidine Tablets, USP does not affect pepsin secretion. Total pepsin output is reduced in proportion to the decrease in volume of gastric juice.
Intrinsic Factor: Oral Ranitidine Tablets, USP has no significant effect on pentagastrin-stimulated intrinsic factor secretion.
Serum Gastrin: Ranitidine Tablets, USP has little or no effect on fasting or postprandial serum gastrin.
Other Pharmacologic Actions:
-
a. Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
-
b. Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
-
c. Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
-
d. No change in cortisol, aldosterone, androgen, or estrogen levels.
-
e. No antiandrogenic action.
-
f. No effect on count, motility, or morphology of sperm.
Pediatrics: Oral doses of 6 to 10 mg/kg per day in two or three divided doses maintain gastric pH>4 throughout most of the dosing interval.
Clinical Trials: Active Duodenal Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed duodenal ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 3
Table 3. Duodenal Ulcer Patient Healing Rates Ranitidine Tablets, USP * Placebo* Number
EnteredHealed /
EvaluableNumber
EnteredHealed /
EvaluableOutpatients 195 69/182
(38%) †188 31-164
(19%)Week 2 Week 4 137/187
(73%) †76/168
(45%)*All patients were permitted p.r.n. antacids for relief of pain.
†P<0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
Table 4. Mean Daily Doses of Antacid Ulcer Healed Ulcer Not Healed Ranitidine 0.06 0.71 Placebo 0.71 1.43 Foreign studies have shown that patients heal equally well with 150 mg b.i.d. and 300 mg h.s. (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg b.i.d. as compared to 300 mg h.s. (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Maintenance Therapy in Duodenal Ulcer: Ranitidine has been found to be effective as maintenance therapy for patients following healing of acute duodenal ulcers. In 2 independent, double-blind, multicenter, controlled trials, the number of duodenal ulcers observed was significantly less in patients treated with Ranitidine Tablets, USP (150 mg h.s.) than in patients treated with placebo over a 12-month period.
Table 5. Duodenal Ulcer Prevalence Double-Blind, Multicenter, Placebo-Controlled Trials Multicenter
TrialDrug Duodenal Ulcer Prevalence No. Of Patients 0-4
Months0-8
Months0-12
MonthsUSA RAN 20%* 24%* 35%* 138 PLC 44% 54% 59% 139 Foreign RAN 12%* 21%* 28%* 174 PLC 56% 64% 68% 165 % = Life table estimate.
* = P<0.05 (Ranitidine Tablets, USP versus comparator).
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H2-antogonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Gastric Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed gastric ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 6.
Table 6. Gastric Ulcer Patient Healing Rates Ranitidine Tablets, USP * Placebo* Number
EnteredHealed /
EvaluableNumber
EnteredHealed /
EvaluableOutpatients 92 16/183
(19%) †94 10/83
(12%)Week 2 Week 6 50/73
(68%) †35/69
(51%)*All patients were permitted p.r.n. antacids for relief of pain.
†P = 0.009.
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Maintenance of Healing of Gastric Ulcers: In two multicenter, double-blind, randomized, placebo-controlled, 12-month trials conducted in patients whose gastric ulcers had been previously healed, Ranitidine Tablets, USP 150 mg h.s. was significantly more effective than placebo in maintaining healing of gastric ulcers.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
Gastroesophageal Reflux Disease (GERD): In 2 multicenter, double-blind, placebo-controlled, 6-week trials performed in the United States and Europe, Ranitidine Tablets, USP 150 mg b.i.d. was more effective than placebo for the relief of heartburn and other symptoms associated with GERD. Ranitidine-treated patients consumed significantly less antacid than did placebo-treated patients.
The US trial indicated that Ranitidine Tablets, USP 150 mg b.i.d. significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In 2 additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg b.i.d. was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
Erosive Esophagitis: In two multicenter, double-blind, randomized, placebo-controlled, 12-week trials performed in the United States, Ranitidine Tablets, USP 150 mg q.i.d. was significantly more effective than placebo in healing endoscopically diagnosed erosive esophagitis and in relieving associated heartburn.
The erosive esophagitis healing rates were as follows:
Table 7. Erosive Esophagitis Patient Healing Rates Healed / Evaluable Placebo*
n=229Ranitidine Tablets, USP
150 mg q.i.d.*
n=215Week 4 43/198 (22%) 96/206 (47%) † Week 8 63/176 (36%) 142/200 (71%) † Week 12 92/159 (58%) 162/192 (84%) † *All patients were permitted p.r.n. antacids for relief of pain.
†P<0.001 versus placebo.
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg q.i.d.
Maintenance of Healing of Erosive Esophagitis: In 2 multicenter, double-blind, randomized, placebo-controlled, 48-week trials conducted in patients whose erosive esophagitis had been previously healed, Ranitidine Tablets, USP 150 mg b.i.d. was significantly more effective than placebo in maintaining healing of erosive esophagitis.
-
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
Ranitidine Tablets, USP is indicated in:
1. Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
2. Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
3. The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
4. Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated.
Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
5. Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
6. Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with Ranitidine Tablets, USP 150 mg b.i.d.
7. Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with Ranitidine Tablets, USP 150 mg q.i.d.
8. Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
- CONTRAINDICATIONS
-
PRECAUTIONS
PRECAUTIONS
General:
1. Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
2. Since Ranitidine Tablets, USP is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (see DOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since Ranitidine Tablets, USP is metabolized in the liver.
3. Rare reports suggest that Ranitidine Tablets, USP may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine Tablets, USP should therefore be avoided in patients with a history of acute porphyria.
- LABORATORY TESTS
-
DRUG INTERACTIONS
Drug Interactions: Although Ranitidine Tablets, USP has been reported to bind weakly to cytochrome P-450 in vitro, recommended doses of the drug do not inhibit the action of the cytochrome P-450-linked oxygenase enzymes in the liver. However, there have been isolated reports of drug interactions that suggest that Ranitidine Tablets, USP may affect the bioavailability of certain drugs by some mechanism as yet unidentified (e.g., a pH-dependent effect on absorption or a change in volume of distribution).
Increased or decreased prothrombin times have been reported during concurrent use of ranitidine and warfarin. However, in human pharmacokinetic studies with dosages of ranitidine up to 400 mg/day, no interaction occurred; ranitidine had no effect on warfarin clearance or prothrombin time. The possibility of an interaction with warfarin at dosages of ranitidine higher than 400 mg/day has not been investigated.
In a ranitidine-triazolam drug-drug interaction study, triazolam plasma concentrations were higher during b.i.d. dosing of ranitidine than triazolam given alone. The mean area under the triazolam concentration-time curve (AUC) values in 18- to 60-year-old subjects were 10% and 28% higher following administration of 75-mg and 150-mg ranitidine tablets, respectively, than triazolam given alone. In subjects older than 60 years of age, the mean AUC values were approximately 30% higher following administration of 75-mg and 150-mg ranitidine tablets. It appears that there were no changes in pharmacokinetics of triazolam and α-hydroxytriazolam, a major metabolite, and in their elimination. Reduced gastric acidity due to ranitidine may have resulted in an increase in the availability of triazolam. The clinical significance of this triazolam and ranitidine pharmacokinetic interaction is unknown.
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of Fertility: There was no indication of tumorigenic or carcinogenic effects in life-span studies in mice and rats at dosages up to 2,000 mg/kg per day.
Ranitidine was not mutagenic in standard bacterial tests (Salmonella, Escherichia coli) for mutagenicity at concentrations up to the maximum recommended for these assays. In a dominant lethal assay, a single oral dose of 1,000 mg/kg to male rats was without effect on the outcome of 2 matings per week for the next 9 weeks.
-
PREGNANCY
Pregnancy: Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in rats and rabbits at doses up to 160 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to ranitidine tablets, USP. There are, however no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- NURSING MOTHERS
-
PEDIATRIC USE
Pediatric Use: The safety and effectiveness of Ranitidine Tablets, USP have been established in the age-group of 1 month to 16 years for the treatment of duodenal and gastric ulcers, gastroesophageal reflux disease and erosive esophagitis, and the maintenance of healed duodenal and gastric ulcer. Use of Ranitidine Tablets, USP in this age-group is supported by adequate and well-controlled studies in adults, as well as additional pharmacokinetic data in pediatric patients and an analysis of the published literature (see CLINICAL PHARMACOLOGY: Pediatrics and DOSADGE AND ADMINISTRATION: Pediatric Use).
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see CLINICAL PHARMACOLOGY: Pediatrics)
-
GERIATRIC USE
Geriatric Use: Of the total number of subjects enrolled in US and foreign controlled clinical trials of oral formulations of Ranitidine Tablets, USP, for which there were subgroup analyses, 4,197 were 65 and over, while 899 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Geriatrics and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function).
-
ADVERSE REACTIONS
ADVERSE REACTIONS
The following have been reported as events in clinical trials or in the routine management of patients treated with Ranitidine Tablets, USP. The relationship to therapy with Ranitidine Tablets, USP has been unclear in many cases. Headache, sometimes severe, seems to be related to administration of Ranitidine Tablets, USP.
Central Nervous System: Rarely, malaise, dizziness, somnolence, insomnia, and vertigo. Rare cases of reversible mental confusion, agitation, depression, and hallucinations have been reported, predominantly in severely ill elderly patients. Rare cases of reversible blurred vision suggestive of a change in accommodation have been reported. Rare reports of reversible involuntary motor disturbances have been received.
Cardiovascular: As with other H2-blockers, rare reports of arrhythmias such as tachycardia, bradycardia, atrioventricular block, and premature ventricular beats.
Gastrointestinal: Constipation, diarrhea, nausea/vomiting, abdominal discomfort/pain, and rare reports of pancreatitis.
Hepatic: There have been occasional reports of hepatocellular, cholestatic, or mixed hepatitis, with or without jaundice. In such circumstances, ranitidine should be immediately discontinued. These events are usually reversible, but in rare circumstances death has occurred. Rare cases of hepatic failure have also been reported. In normal volunteers, SGPT values were increased to at least twice the pretreatment levels in 6 of 12 subjects receiving 100 mg q.i.d. intravenously for 7 days, and in 4 of 24 subjects receiving 50 mg q.i.d. intravenously for 5 days.
Musculoskeletal: Rare reports of arthralgias and myalgias.
Hematologic: Blood count changes (leucopenia, granulocytopenia, and thrombocytopenia) have occurred in a few patients. These were usually reversible. Rare cases of agranulocytosis, pancytopenia, sometimes with marrow hypoplasia, and aplastic anemia and exceedingly rare cases of acquired immune hemolytic anemia have been reported.
Endocrine: Controlled studies in animals and man have shown no stimulation of any pituitary hormone by Ranitidine Tablets, USP and no antiandrogenic activity, and cimetidine-induced gynecomastia and impotence in hypersecretory patients have resolved when Ranitidine Tablets, USP has been substituted. However, occasional cases of gynecomastia, impotence, and loss of libido have been reported in male patients receiving Ranitidine Tablets, USP, but the incidence did not differ from that in the general population.
Rares cases of breast symptoms and conditions, including galactorrhea and gynecomastia, have been reported in both males and females.
Integumentary: Rash, including rare cases of erythema multiforme. Rare cases of alopecia and vasculitis.
Respiratory: A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H2RAs) compared to patients who had stopped H2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07 - 2.48). However, a causal relationship between use of H2RAs and pneumonia has not been established.
Other: Rare cases of hypersensitiviy reactions (e.g., bronchospasm, fever, rash, eosinophilia), anaphylaxis, angioneurotic edema, and small increases in serum creatinine.
-
OVERDOSAGE
OVERDOSAGE
There has been limited experience with overdosage. Reported acute ingestions of up to 18 g orally have been associated with transient adverse effects similar to those encountered in normal clinical experience (see ADVERSE REACTIONS). In addition, abnormalities of gait and hypotension have been reported.
When overdosage occurs, the usual measures to remove unabsorbed material from the gastrointestinal tract, clinical monitoring, and supportive therapy should be employed.
Studies in dogs receiving dosages of Ranitidine Tablets, USP in excess of 225 mg/kg per day have shown muscular tremors, vomiting, and rapid respiration. Single oral doses of 1,000 mg/kg in mice and rats were not lethal. Intravenous LD50 values in mice and rats were 77 and 83 mg/kg, respectively.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer: The current recommended adult dosage of Ranitidine Tablets, USP for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal or at bedtime can be used for patients in whom dosing convenience is important. The advantages of one treatment regimen compared to the other in a particular patient population have yet to be demonstrated (see Clinical Trials: Active Duodenal Ulcer). Smaller doses have been shown to be equally effective in inhibiting gastric acid secretion in US studies, and several foreign trials have shown that 100 mg twice daily is as effective as the 150-mg dose.
Antacid should be given as needed for relief of pain (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Maintenance of Healing of Duodenal Ulcers: The current recommended adult oral dosage is 150 mg at bedtime.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
The current recommended adult oral dosage is 150 mg twice a day. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
Benign Gastric Ulcer: The current recommended adult oral dosage is 150 mg twice a day.
Maintenance of Healing of Gastric Ulcers: The current recommended adult oral dosage is 150 mg at bedtime.
GERD: The current recommended adult oral dosage is 150 mg twice a day.
Erosive Esophagitis: The current recommended adult oral dosage is 150 mg four times a day.
Maintenance of Healing of Erosive Esophagitis: The current recommended adult oral dosage is 150 mg twice a day.
Pediatric Use: The safety and effectiveness of Ranitidine Tablets, USP have been established in the age-group of 1 month to 16 years. There is insufficient information about the pharmacokinetics of Ranitidine Tablets, USP in neonatal patients (less than 1 month of age) to make dosing recommendations.
The following 3 subsections provide dosing information for each of the pediatric indications.
Treatment of Duodenal and Gastric Ulcers: The recommended oral dose for the treatment of active duodenal and gastric ulcers is 2 to 4 mg/kg twice daily to a maximum of 300 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Maintenance of Healing of Duodenal and Gastric Ulcers: The recommended oral dose for the maintenance of healing of duodenal and gastric ulcers is 2 to 4 mg/kg once daily to a maximum of 150 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Treatment of GERD and Erosive Esophagitis: Although limited data exist for these conditions in pediatric patients, published literature supports a dosage of 5 to 10 mg/kg per day, usually given as two divided doses.
Dosage Adjustment for Patients With Impaired Renal Function: On the basis of experience with a group of subjects with severely impaired renal function treated with Ranitidine Tablets, USP, the recommended dosage in patients with a creatinine clearance more than 50 mL/min is 150 mg every 24 hours. Should the patient's condition require, the frequency of dosing may be increased to every 12 hours or even further with caution. Hemodialysis reduces the level of circulating ranitidine. Ideally, the dosing schedule should be adjusted so that the timing of a scheduled dose coincides with the end of hemodialysis.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Geriatrics and PRECAUTIONS: Geriatric Use).
-
HOW SUPPLIED
HOW SUPPLIED
Ranitidine Tablets, USP 150 mg are orange, round, biconvex aqueous film coated tablets debossed “IP 253” on one side and plain on the reverse.
They are available in bottles of 24, 60, 100, 180, 500 and 1000.
Ranitidine Tablets, USP 300 mg are yellow, capsule-shaped aqueous film coated tablets debossed “IP 254” on one side and plain on the reverse.
They are available in bottles of 24, 30, 100, 250, 500 and 1000.
Store at 20° - 25°C (68° - 77°F) (See USP Controlled Room Temperature) in a tight, light resistant container. Protect from light. Replace cap securely after each opening.
Manufactured by:
Amneal Pharmaceuticals of NY
Hauppauge, NY 11788
Distributed by:
Amneal Pharmaceuticals
Glasgow, KY 42141
Rev. 11-2009
- PRINCIPAL DISPLAY PANEL
-
SPL UNCLASSIFIED SECTION
Sentra PM™PRODUCT INFORMATION Sentra PM (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes of sleep disorders (SD). Must be administered under physician supervision. Medical Foods Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a system which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology. Sentra PM has been developed, manufactured, and labeled in accordance with both the statutory and the FDA regulatory definition of a Medical Food. Sentra PM must be used while the patient is under the ongoing care of a physician. SLEEP DISORDERS (SD) SD as a Metabolic Deficiency Disease A critical component of the definition of a Medical Food is the requirement for a distinctive nutritional deficiency. FDA scientists have proposed a physiologic definition of a distinctive nutritional deficiency as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflect the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism, and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with sleep disorders responds to a tryptophan formulation by improving the duration and quality of sleep, a deficiency of tryptophan is assumed to exist. Patients with sleep disorders are known to have nutritional deficiencies of tryptophan, choline, flavonoids, and certain antioxidants. Patients with sleep disorders frequently exhibit reduced plasma levels of tryptophan and have been shown to respond to oral administration of tryptophan or a 5-hydoxytryptophan formulation. Research has shown that tryptophan reduced diets result in a fall of circulating tryptophan. Patients with sleep disorders have activation of the tryptophan degradation pathway that increases the turnover of tryptophan leading to a reduced level of production of serotonin for a given tryptophan blood level. Research has also shown that a genetic predisposition can lead to increased tryptophan requirements in certain patients with sleep disorders. Choline is required to fully potentiate acetylcholine synthesis by brain neurons. A deficiency of choline leads to reduced acetylcholine production by the neurons. Low fat diets, frequently used by patients with sleep disorders, are usually choline deficient. Flavonoids potentiate the production of acetylcholine by the neurons thereby inducing REM sleep. Low fat diets and diets deficient in flavonoid rich foods result in inadequate flavonoid concentrations, impeding acetylcholine production in certain patients with sleep disorders. Provision of tryptophan, choline, and flavonoids with antioxidants, in specific proportions can restore the production of beneficial serotonin and acetylcholine, thereby improving sleep quality.
-
DESCRIPTION
PRODUCT DESCRIPTION Primary Ingredients Sentra PM consists of a proprietary blend of amino acids, cocoa, ginkgo biloba and flavonoids in specific proportions. These ingredients fall into the category of “Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186. Amino Acids Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for thousands of years. The doses of the amino acids in Sentra PM are equivalent to those found in the usual human diet; however the formulation uses specific ratios of the key ingredients to elicit a therapeutic response. Patients with sleep disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to induce sleep. Patients with sleep disorders have altered serotonin metabolism. Some patients with sleep disorders have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance that is genetically determined. Patients with sleep disorders frequently cannot acquire sufficient tryptophan from the diet without ingesting a prohibitively large amount of calories, particularly protein rich calories. Flavonoids Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Sentra PM cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response. Other Ingredients Sentra PM contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material). Physical Description Sentra PM is a yellow to light brown powder. Sentra PM contains L-Glutamic Acid, 5-Hydroxytryptophan as Griffonia Seed Extract, Acetylcarnitine HCL, Choline Bitartrate, Cinnamon, Cocoa, Ginkgo Biloba, and Hawthorn Berry.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY Mechanism of Action Sentra PM acts by restoring and maintaining the balance of the neurotransmitters, serotonin and acetylcholine, that are associated with sleep disorders. Metabolism The amino acids in Sentra PM are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Sentra PM. Circulating tryptophan and choline blood levels determine the production of serotonin and acetylcholine. Excretion Sentra PM is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
- INDICATIONS & USAGE
-
CLINICAL STUDIES
CLINICAL EXPERIENCE The administration of Sentra PM has demonstrated significant functional improvement in the quality and quantity of sleep when used for the dietary management of the metabolic processes associated with sleep disorders. Administration of Sentra PM results in the induction and maintenance of sleep in patients with sleep disorders. Sentra PM has no effect on normal blood pressure.
- PRECAUTIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS Oral supplementation with L-tryptophan or choline at high doses up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Some patients may experience these symptoms at lower doses. The total combined amount of amino acids in each Sentra PM capsule does not exceed 400 mg.
- DRUG INTERACTIONS
-
OVERDOSAGE
OVERDOSE There is a negligible risk of overdose with Sentra PM as the total dosage of amino acids in a one month supply (60 capsules) is less than 24 grams. Overdose symptoms may include diarrhea, weakness, and nausea. POST-MARKETING SURVEILLANCE Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Sentra PM flavonoid ingredients, including cinnamon, cocoa, and chocolate. The reactions were transient in nature and subsided within 24 hours.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION Recommended Administration For the dietary management of the metabolic processes associated with sleep disorders. Take (2) capsules daily at bedtime. An additional dose of one or two capsules may be taken after awakenings during the night. As with most amino acid formulations Sentra PM should be taken without food to increase the absorption of key ingredients.
-
HOW SUPPLIED
How Supplied Sentra PM is supplied in red and white, size 0 capsules in bottles of 60 capsules. Physician Supervision Sentra PM is a Medical Food product available by prescription only and must be used while the patient is under ongoing physician supervision. Sentra PM is supplied to physicians in a recyclable plastic bottle with a child-resistant cap. U.S. patents pending. Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225 Distributed by Physician Therapeutics LLC, Los Angeles, CA 90077. www.ptlcentral.com © Copyright 2003-2006, Physician Therapeutics LLC, all rights reserved NDC # 68405-1003-02
- STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
PHYSICIAN THERAPEUTICS SENTRA PM Medical Food Rx only 60 Capsules Directions for use: Must be administered under medical supervision. For adults only. As a Medical Food, take two (2) capsules at bedtime or as directed by your medical practitioner. For the dietary management of sleep disorders. Contains no added sugar, starch, wheat, yeast, preservatives, artificial color or flavor. Storage: Keep tightly closed in a cool dry place 8-320 C (45-900F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-1003-02 Ingredients: Each serving (2 capsules) contains: Proprietary Amino Acid blend Choline Bitartrate, Glutamic Acid (L-Glutamic Acid), Cocoa Extract (fruit), Proprietary Herbal Blend Ginkgo Biloba (leaves), Griffonia Seed Extract (5-HTP), Hawthorn Berry (fruit), Acetyl L-Carnitine HCl, Dextrose Other Ingredients: Gelatin, Cellulose, Dicalcium Phosphate, Silicon Dioxide and Vegetable Magnesium Stearate. Distributed by: Physician Therapeutics LLC, Los Angeles, CA 90077 www.ptlcentral.com Patent Pending
-
PRINCIPAL DISPLAY PANEL
For the Dietary Management of Sleep Disorders. Two capsules at bedtime or as directed by physician. See product label and insert. Sentra PM Medical Food A Convenlence Packed Medical Food And Drug Sentradine PHYSICIAN THERAPEUTICS > Sentra PM 60 Capsules > Ranitidine 150 mg 30 Tablets No Refills Without Physician Authorization Rx only NDC# 68405-8033-26 of this co-pack FRONT VIEW As prescribed by physician. See product label and product information insert. Ranitidine 150 mg Rx Drug BACK VIEW Physician Therapeutics LLC Los Angeles, CA 90077 on November 21, 2006
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENTRADINE
ranitidine hydrochloride, choline kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68405-033 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68405-033-26 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 Part 2 1 BOTTLE 60 Part 1 of 2 RANITIDINE
ranitidine hydrochloride tabletProduct Information Item Code (Source) NDC:52959-502(NDC:53746-253) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE HYDROCHLORIDE 150 mg Product Characteristics Color orange (ORANGE) Score no score Shape ROUND (Biconvex) Size 9mm Flavor Imprint Code IP253 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-502-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077824 07/07/2011 Part 2 of 2 SENTRA PM
choline capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLINE (UNII: N91BDP6H0X) (CHOLINE - UNII:N91BDP6H0X) CHOLINE 250 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MALTODEXTRIN (UNII: 7CVR7L4A2D) GELATIN (UNII: 2G86QN327L) Product Characteristics Color red (RED) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Medical Food 07/07/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/07/2011 Labeler - Physician Therapeutics LLC (931940964) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals 831227801 manufacture Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 repack Establishment Name Address ID/FEI Business Operations Targeted Medical Pharma, Inc. 126962740 manufacture