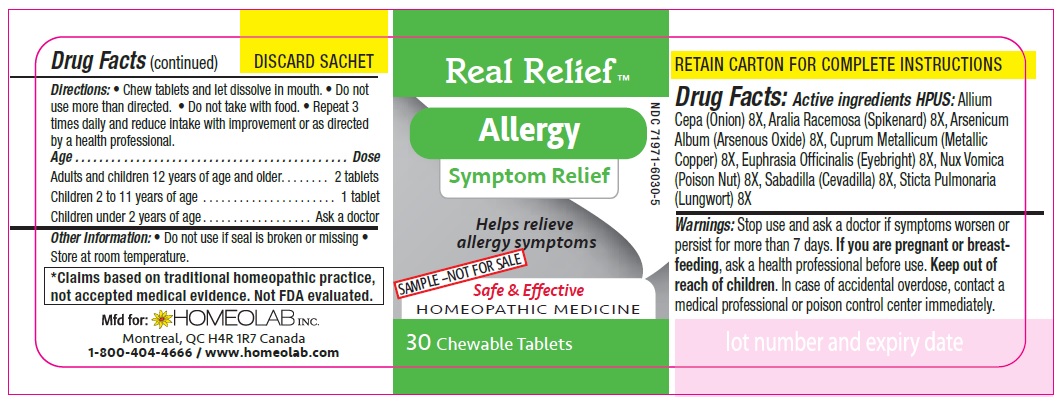
Label: REAL RELIEF- allium cepa, aralia racemosa, arsenicum album, cuprum metallicum, euphrasia officinalis, nux vomica, sabadilla, sticta pulmonaria tablet
- NDC Code(s): 71971-6030-0, 71971-6030-5, 71971-6030-8
- Packager: Homeolab International (Canada) inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
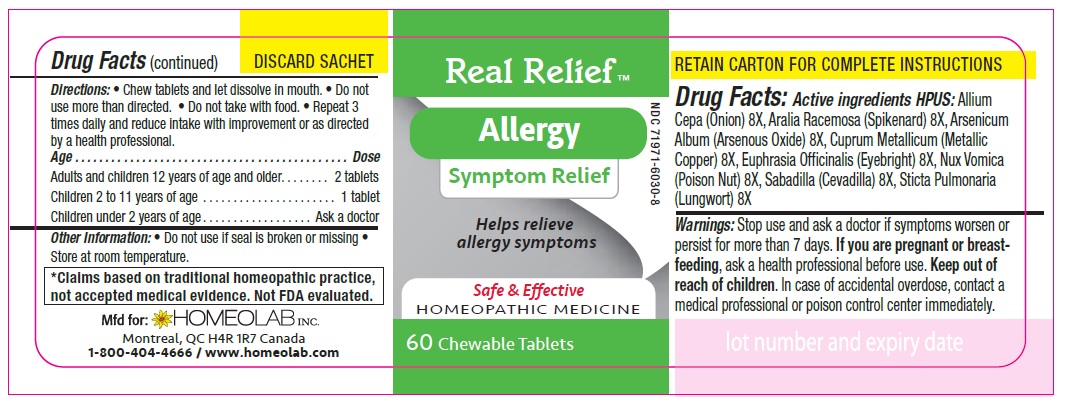
ACTIVE INGREDIENT
Active Ingredients HPUS:
Allium cepa (Onion) 8 [hp-X]
Aralia racemosa (Spikenard) 8 [hp-X]
Arsenicum album (Arsenous trioxyde) 8 [hp-X]
Cuprum metallicum (Metallic copper) 8 [hp-X]
Euphrasia officinalis (Eyebright) 8 [hp-X]
Nux vomica (Poison nut) 8 [hp-X]
Sabadilla (Sabadilla) 8 [hp-X]
Sticta pulmonaria (Lungwort) 8 [hp-X]
-
PURPOSE
Purpose
Homeopathic remedy helps relieve symptoms of all types of allergies:
- itchy, watery eyes
- red burning eyes and sneezing
- nasal congestion, coryza
- burning and swelling of the eye lids
- sneezing, runny nose, hay fever
- hay fever, sneezing, runny nose
- dry cough, hay fever
- itchy eyes, nasal congestion
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 3 times daily and reduce intake with improvement or as directed by a health professional
Age…………………………………………………………........ Dose
Adults and Children 12 years of age and older………...... 2 tablets
Children 2 to 11 years of age…...……………………............ 1 tablet
Children under 2 years of age……………………................. Ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Product label
-
INGREDIENTS AND APPEARANCE
REAL RELIEF
allium cepa, aralia racemosa, arsenicum album, cuprum metallicum, euphrasia officinalis, nux vomica, sabadilla, sticta pulmonaria tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71971-6030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 8 [hp_X] ARALIA RACEMOSA ROOT (UNII: T90W4582DU) (ARALIA RACEMOSA ROOT - UNII:T90W4582DU) ARALIA RACEMOSA ROOT 8 [hp_X] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 8 [hp_X] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 8 [hp_X] EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 8 [hp_X] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 8 [hp_X] SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 8 [hp_X] LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 8 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code HLB Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71971-6030-0 1 in 1 CARTON 01/01/2020 1 90 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:71971-6030-5 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2020 3 NDC:71971-6030-8 1 in 1 CARTON 01/01/2020 3 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2020 Labeler - Homeolab International (Canada) inc (203639455)