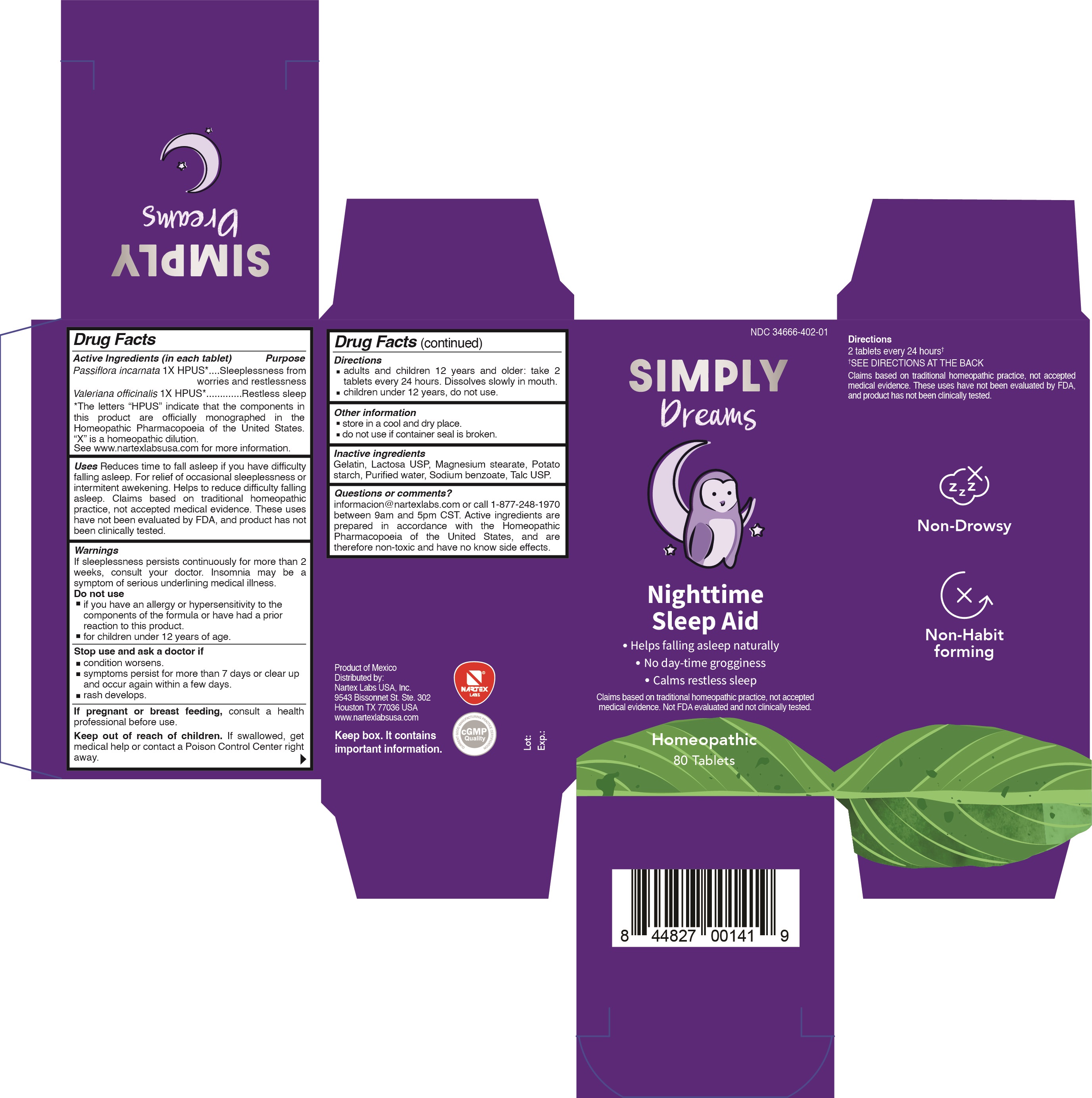
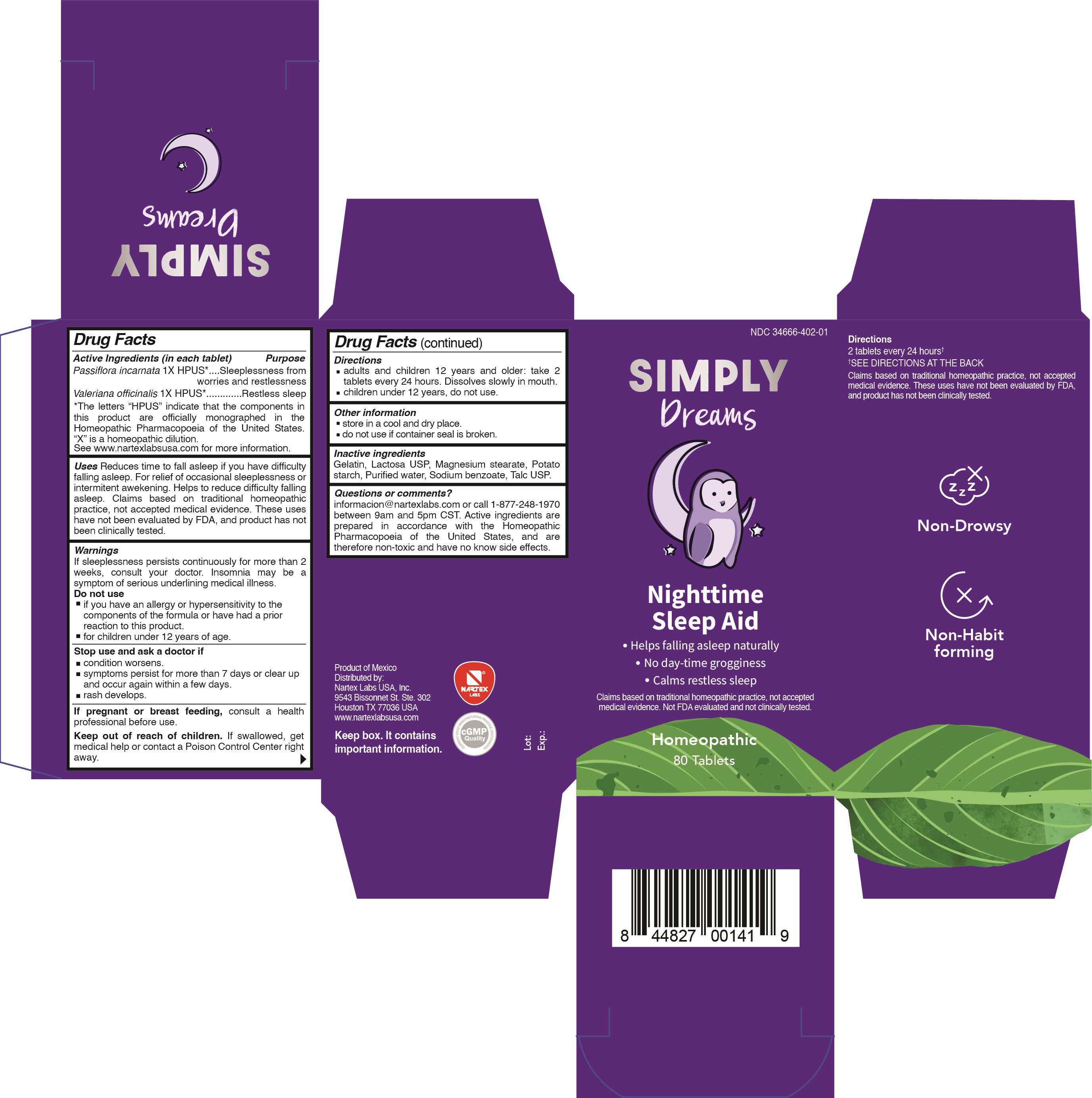
Label: SIMPLY DREAMS- passiflora incarnata, valeriana officinalis tablet
- NDC Code(s): 34666-402-01
- Packager: Nartex Laboratorios Homeopaticos, S.A. De C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 3, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
-
Purpose
Passiflora incarnata 1X HPUS*...................Sleeplessness from worries and restlessness
Valeriana officinalis 1X HPUS*....................Restless sleep
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. "X" is a homeopathic dilution. See www.nartexlabsusa.com for more information.
-
Uses
Reduces time to fall asleep if you have difficulty falling asleep. For relief of occasional sleeplessness or intermittent awakening. Helps to reduce difficulty falling asleep. Claims based on traditional homeopathic practice, not accepted medical evidence. These uses have not been evaluated by FDA, and product has not been clinically tested.
-
Warnings
If sleeplessness persists continuously for more than 2 weeks, consult your doctor. Insomnia may be a symptom of serious underlying medical illness.
Do not use
- if you have an allergy or hypersensitivity to the components of the formula or have had a prior reaction to this product.
- for children under 12 years of age.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SIMPLY DREAMS
passiflora incarnata, valeriana officinalis tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34666-402 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 1 [hp_X] VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 1 [hp_X] Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) GELATIN (UNII: 2G86QN327L) STARCH, POTATO (UNII: 8I089SAH3T) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white Score score with uneven pieces Shape ROUND Size 9mm Flavor Imprint Code LogoNartex Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34666-402-01 1 in 1 CARTON 12/03/2021 1 80 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/03/2021 Labeler - Nartex Laboratorios Homeopaticos, S.A. De C.V. (589914576) Establishment Name Address ID/FEI Business Operations Nartex Laboratorios Homeopaticos, S.A. De C.V. 589914576 manufacture(34666-402)