Label: EPSOM SALT granule
- NDC Code(s): 46122-461-19, 46122-461-43
- Packager: AmerisourceBergen (Good Neighbor Pharmacy) 46122
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that lasts more than 2 weeks
-
Directions
- do not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours
age dose adults and children 12 years and older 2-4 level teaspoons dissolved in a full glass (8oz) of water children 6 to 11 years 1-2 level teaspoons dissolved in a full glass (8oz) of water
Not recommended for children under 6 years of age. - Other information
- Inactive ingredient
- Questions or comments?
-
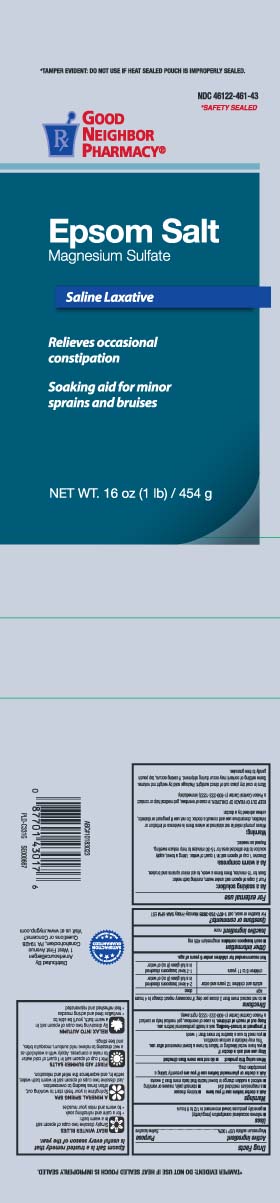
Principal Display Panel
*SAFETY SEALED
Epsom Salt
Magnesium Sulfate
Saline Laxative
Relieves occasional constipation
Soaking aid for minor sprains and bruises
Net Wt LB (kg)
*TAMPER EVIDENT: DO NOT USE IF HEAT SEALED POUCH IS IMPROPERLY SEALED
Distributed by: AmerisouceBergen
1 West First Avenue
Conshohocken, PA 19428
Visit us at www.mygnp.com
- Package Label
-
INGREDIENTS AND APPEARANCE
EPSOM SALT
epsom salt granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-461 Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE HEPTAHYDRATE 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-461-19 1814 g in 1 POUCH; Type 0: Not a Combination Product 04/30/2018 2 NDC:46122-461-43 454 g in 1 POUCH; Type 0: Not a Combination Product 05/30/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 04/30/2018 Labeler - AmerisourceBergen (Good Neighbor Pharmacy) 46122 (007914906)