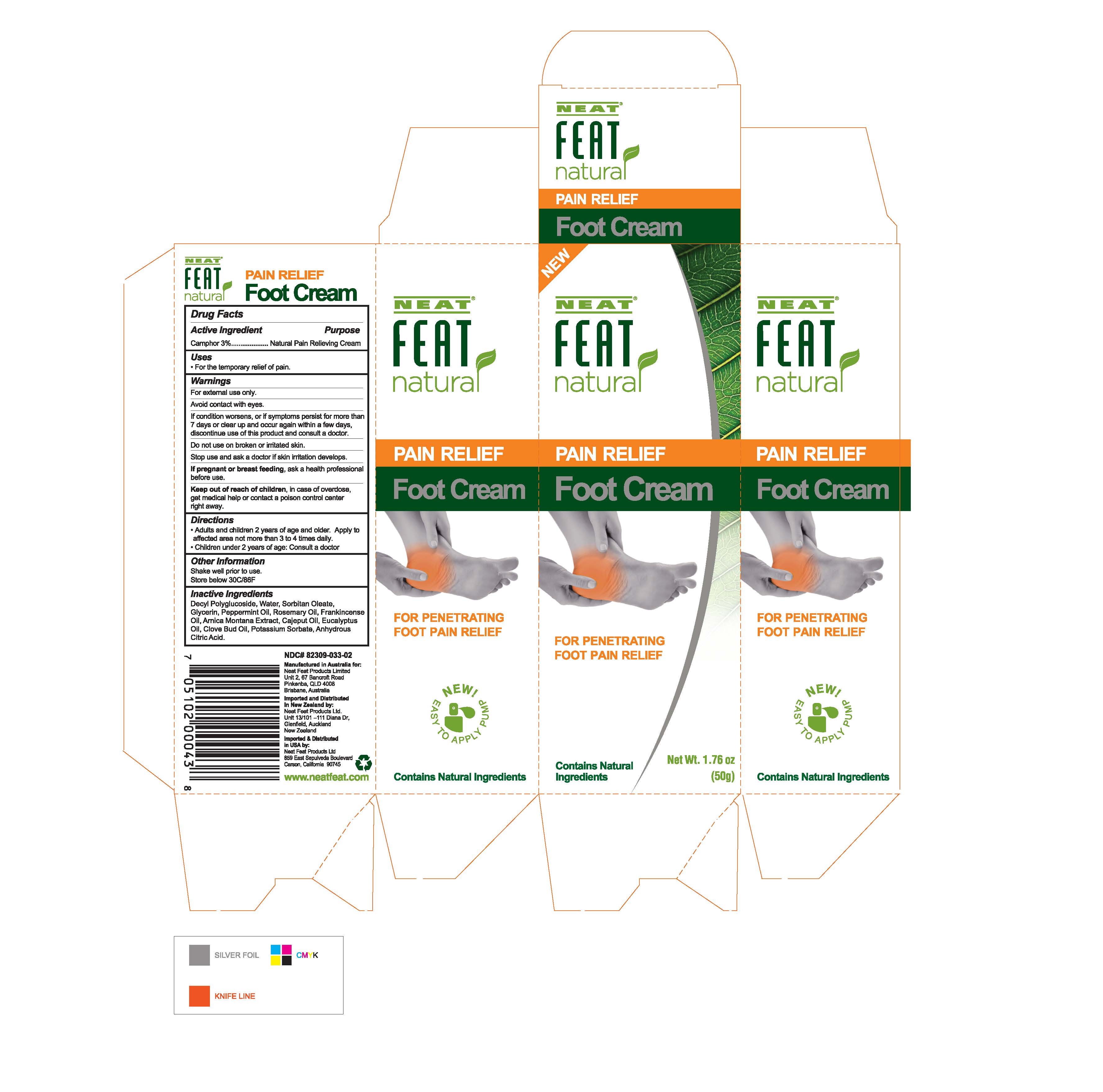
Label: NATURAL PAIN RELIEF FOOT CREAM- camphor cream
- NDC Code(s): 82309-033-01, 82309-033-02
- Packager: Tripak Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Natural Foot Cream Box
-
INGREDIENTS AND APPEARANCE
NATURAL PAIN RELIEF FOOT CREAM
camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82309-033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 1.5 g in 50 g Inactive Ingredients Ingredient Name Strength PEG-6 SORBITAN OLEATE (UNII: 58O7V09UCI) GLYCERIN (UNII: PDC6A3C0OX) ROSEMARY OIL (UNII: 8LGU7VM393) EUCALYPTUS OIL (UNII: 2R04ONI662) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) WATER (UNII: 059QF0KO0R) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) PEPPERMINT OIL (UNII: AV092KU4JH) FRANKINCENSE OIL (UNII: 67ZYA5T02K) ARNICA MONTANA (UNII: O80TY208ZW) CAJUPUT OIL (UNII: J3TO6BUQ37) CLOVE OIL (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82309-033-02 1 in 1 BOX 12/01/2021 1 NDC:82309-033-01 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2019 Labeler - Tripak Pharmaceuticals (758658819)