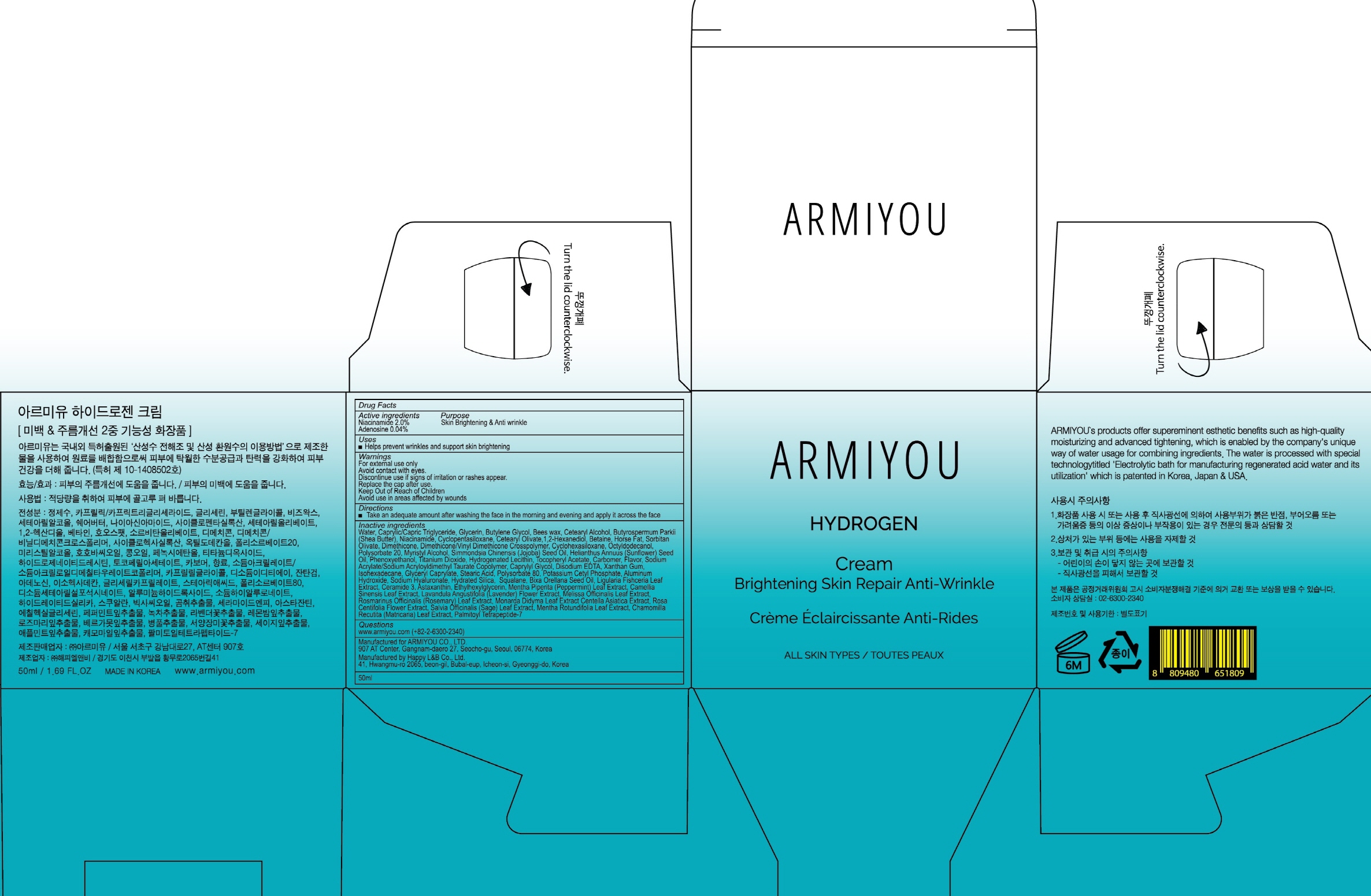
Label: ARMIYOU HYDROGEN- niacinamide, adenosine cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 71878-040-01, 71878-040-02 - Packager: Armiyou
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 22, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients: Water, Caprylic/Capric Triglyceride, Glycerin, Butylene Glycol, Bees wax, Cetearyl Alcohol, Butyrospermum Parkii (Shea Butter), Niacinamide, Cyclopentasiloxane, Cetearyl Olivate,1,2-Hexanediol, Betaine, Horse Fat, Sorbitan Olivate, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Cyclohexasiloxane, Octyldodecanol, Polysorbate 20, Myristyl Alcohol, Simmondsia Chinensis (Jojoba) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Phenoxyethanol, Titanium Dioxide, Hydrogenated Lecithin, Tocopheryl Acetate, Carbomer, Flavor, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Caprylyl Glycol, Disodium EDTA, Xanthan Gum, Isohexadecane, Glyceryl Caprylate, Stearic Acid, Polysorbate 80, Potassium Cetyl Phosphate, Aluminum Hydroxide, Sodium Hyaluronate, Hydrated Silica, Squalane, Bixa Orellana Seed Oil, Ligularia Fishceria Leaf Extract, Ceramide 3, Astaxanthin, Ethylhexylglycerin, Mentha Piperita (Peppermint) Leaf Extract, Camellia Sinensis Leaf Extract, Lavandula Angustifolia (Lavender) Flower Extract, Melissa Officinalis Leaf Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Monarda Didyma Leaf Extract Centella Asiatica Extract, Rosa Centifolia Flower Extract, Salvia Officinalis (Sage) Leaf Extract, Mentha Rotundifolia Leaf Extract, Chamomilla Recutita (Matricaria) Leaf Extract, Palmitoyl Tetrapeptide-7
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARMIYOU HYDROGEN
niacinamide, adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71878-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 1.00 g in 50 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.02 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71878-040-02 1 in 1 CARTON 10/01/2017 1 NDC:71878-040-01 50 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2017 Labeler - Armiyou (694891814) Registrant - Armiyou (694891814) Establishment Name Address ID/FEI Business Operations Armiyou 694891814 manufacture(71878-040)